(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured a session on kidney cancer trials in progress, and a presentation by Dr. Eric Jonasch discussing the trial design of a phase 1b/2 study of combination 177Lu girentuximab + cabozantinib and nivolumab in treatment-naive patients with advanced clear cell RCC.
Complete response is still a rare event in patients with advanced clear cell renal cell carcinoma. The combination of nivolumab + cabozantinib was recently approved for the first-line treatment of clear cell renal cell carcinoma based on the CheckMate 9ER phase 3 study demonstrating improved progression-free survival and objective response rate in comparison to sunitinib.1 However, the complete response rate in CheckMate 9ER was only 9%.
Since the anti-tumor effects of immune checkpoint inhibitors are dependent on the presence of activated tumor-infiltrating T cells, drugs that could synergize with T cells' anti-tumor activity can allow us to improve complete response rates. Activation of the cGAS-STING pathway which is induced by radiation-induced DNA damage, is one promising mechanism that has been investigated. Many studies have shown that radiation treatment augments immune checkpoint inhibition. However, it is not always possible to radiate all metastatic lesions. Therefore, targeted peptide receptor radionuclide therapies, have been developed by conjugating radioisotopes to receptor binding analogs targeting specific cancer cell surface proteins, thereby delivering targeted radiation to cancer cells in the body with minimal damage to surrounding healthy cells: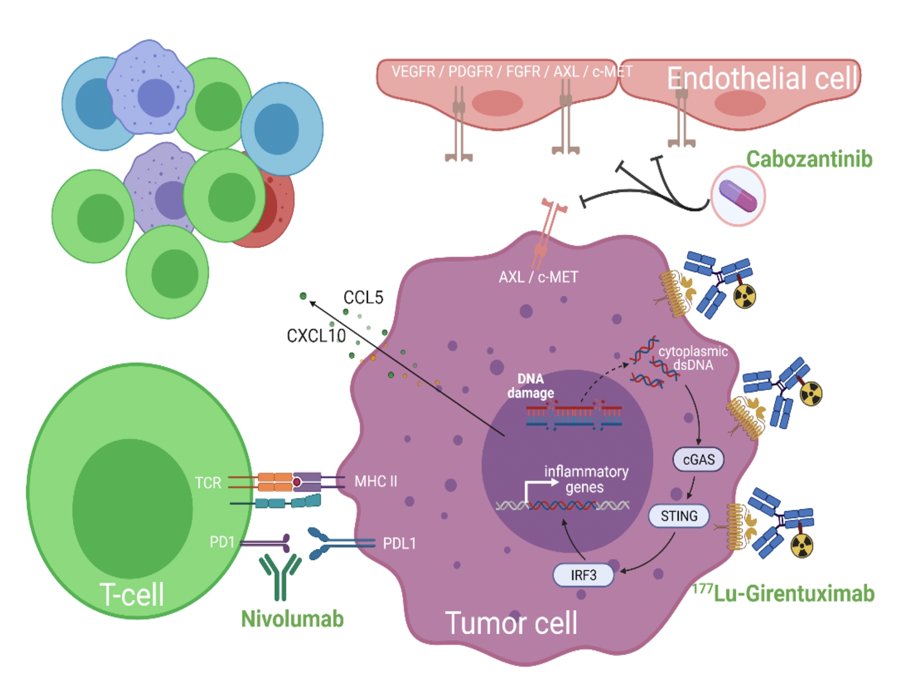
177Lu girentuximab is the first antibody-radioisotope designed for clear cell renal cell carcinoma, targeting carbonic anhydrase 9-expressing cells, which includes >90% of clear cell renal cell carcinoma. It has been tested in metastatic clear cell renal cell carcinoma as a single agent and shown to be safe and effective in stabilizing disease in 57% of patients. In this study, Dr. Jonasch and colleagues hypothesize that 177Lu girentuximab-induced DNA damage will potentiate the STING pathway, and this activation will synergize with nivolumab and cabozantinib to promote trafficking and infiltration of activated T cells to tumors and achieve higher complete response rates.
This is a single-arm, phase 1b/2 study that will test the hypothesis that adding 177Lu girentuximab to cabozantinib + nivolumab will increase the complete response rate of therapy will increase the complete response rate when compared to historical outcomes with the nivolumab + cabozantinib doublet. The primary objective is to determine the safety and complete response rate of combination 177Lu girentuximab + nivolumab and cabozantinib in subjects with previously untreated clear cell renal cell carcinoma. 177Lu-girentuximab 1480 MBq/m2 (61% of single agent maximum tolerated dose) will be administered every 12 weeks for up to 3 cycles: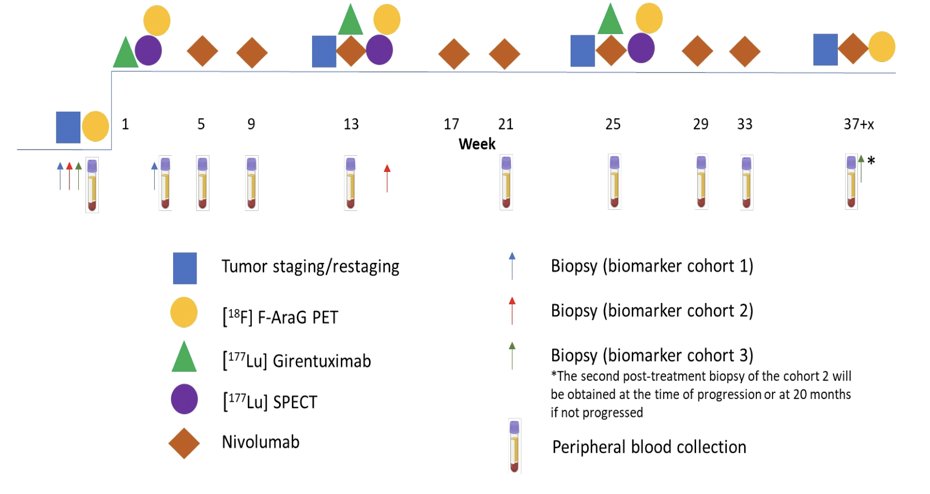
Starting with the second cycle, nivolumab, and cabozantinib will be added at standard dose. To explore the effects of the treatment on inducing activated T cell infiltration, patients will undergo pre/post-treatment PET scan with 18F-AraG radiotracer as well as biopsies for single cell, spatial transcriptomics, and proteomics studies.
Up to 100 patients with treatment-naive, biopsy-proven clear cell renal cell carcinoma with adequate organ/marrow function with one evaluable lesion by RECIST 1.1 will be enrolled. A 5-patient safety lead-in will evaluate myelosuppression. Ongoing safety and futility monitoring will employ a Bayesian approach. The sample size was chosen for reasonable operating characteristics to distinguish a complete response rate (primary endpoint) of 18% as better than 9% using a beta (0.09, 0.91) prior. Secondary endpoints are objective response rate, progression-free survival by RECIST 1.1, duration of response, clinical benefit, tumor response, and overall survival.
Clinical trial information: NCT05663710.
Presented: Eric Jonasch, MD, Professor, Department of Genitourinary Medical Oncology, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
References:


