(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31 and June 4 was host to the Session: Managing Variant Histologies in Urothelial and Renal Cell Cancers. Dr. Sumanta Kumar Pal discussed What Is Variant Histology Renal Cell Cancer and What Are the Available Treatment Options to date.
Approximately 20-25% of renal cell carcinoma (RCC) cases are non-clear cell subtypes, with the majority being papillary. This distinction is crucial as it impacts treatment decisions and prognostic outcomes for patients with RCC. Clear cell RCC has historically received more attention in research and treatment protocols, but understanding and addressing non-clear cell subtypes is essential for providing optimal care to patients. There have been randomized phase Il trials in papillary RCC, and single-arm studies in exquisitely rare histologies have been successfully conducted (collecting duct, renal medullary cancer) but optimal therapy is debatable.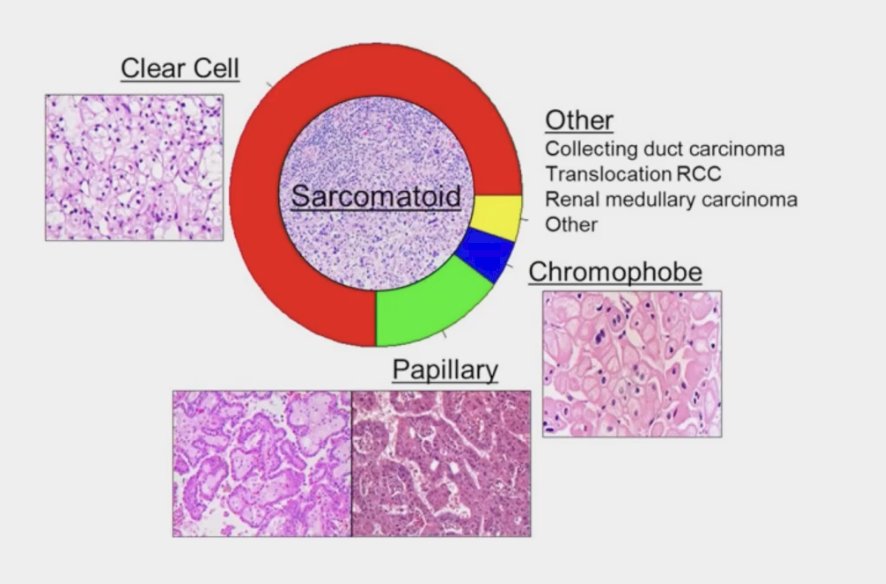
The table below provides a comprehensive summary of the genes implicated in hereditary syndrome renal cell carcinoma (RCC), along with their corresponding inheritance patterns. Dr. Kumar Pal emphasized the significance of this study recently published by Naik and collaborators, underscoring the importance of understanding the genetic underpinnings of hereditary RCC.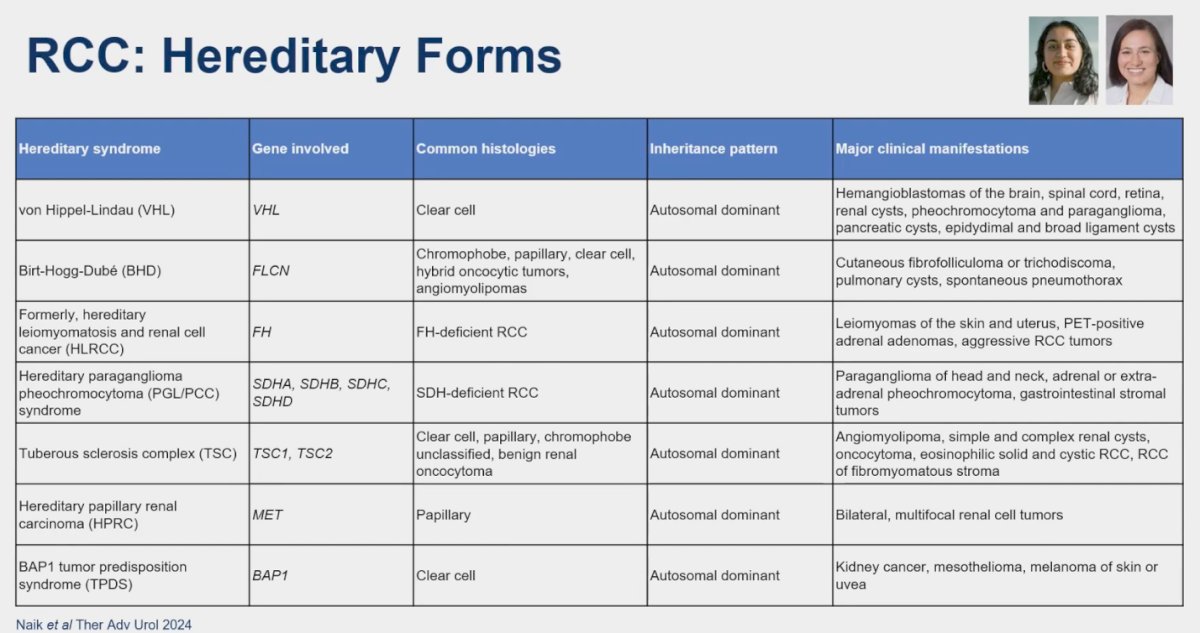
Papillary RCC (pRCC)
The majority of non-clear cell renal cell carcinoma (RCC) cases belong to the papillary subtype (pRCC). Dr. Kumar Pal provided an overview of randomized phase II trials and single-arm studies conducted on this subtype, offering insights into the evolving landscape of treatment options for patients with pRCC.
The PAPMET (SWOG 1500) clinical trial randomized patients with pRCC who have received at least one line of therapy to Sunitinib, Cabozantinib, Crizotinib, and Savolitinib. This trial aimed to leverage the underlying pathology of pRCC, particularly targeting the MET oncogene. The study showed a significant PFS advantage with Cabozantinib vs. Sunitinib ( 9 vs. 5.6 months, p=0.019) but no overall survival (OS) advantage. Interestingly, the arms of the study involving more specific MET inhibitors, such as Crizotinib and Savolitinib, experienced early discontinuation during a futility analysis. Despite being a small randomized controlled trial with a sample size (n=45) in the Cabozantinib arm, raises the question of Cabozantinib emerged as the standard of care for pRCC.1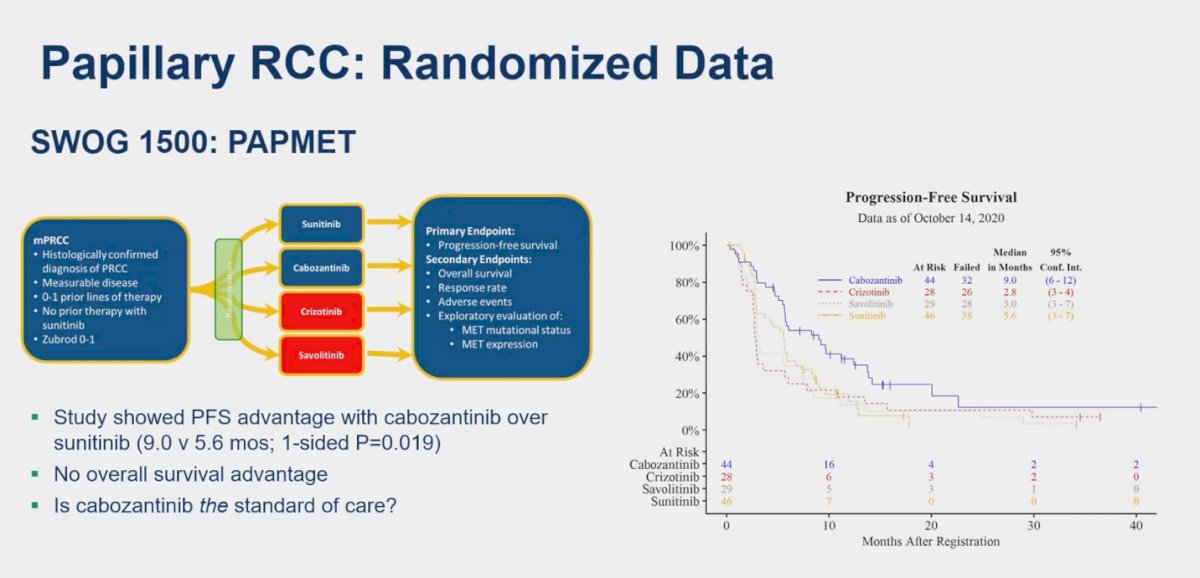
In a single-arm study involving 32 patients with papillary RCC (ppRCC), the combination of Cabozantinib and Atezolizumab demonstrated a notable response rate (RR) of 47%.2 Additionally, another single-arm study conducted at the Memorial Sloan Kettering Cancer Center (MSKCC) evaluated the efficacy of Cabozantinib in combination with Nivolumab in 40 patients with pRCC. According to RECIST 1.1 criteria, this regimen yielded an objective response rate (ORR) of 48%.3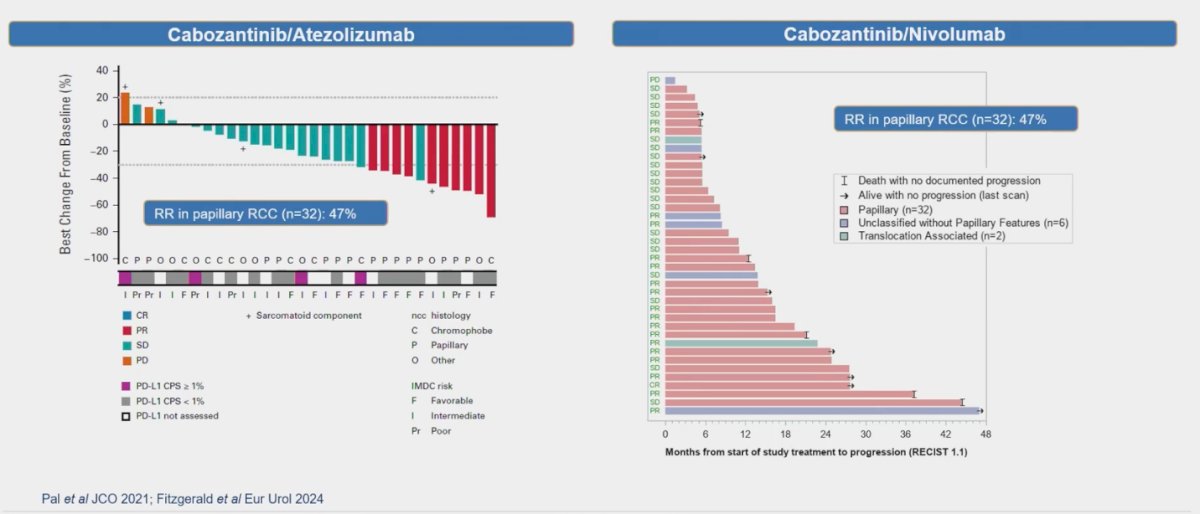
Several other studies have explored combinations of VEGF inhibitors with immunotherapy (IO), such as Bevacizumab in combination with Atezolizumab. In a small cohort of pRCC patients (n=12), this regimen reported a response rate (RR) of 25%.4 Similarly, Lenvatinib a multitargeted TKI combined with Pembrolizumab was investigated in a larger cohort of 93 patients with pRCC, showing a remarkable RR of 54%.5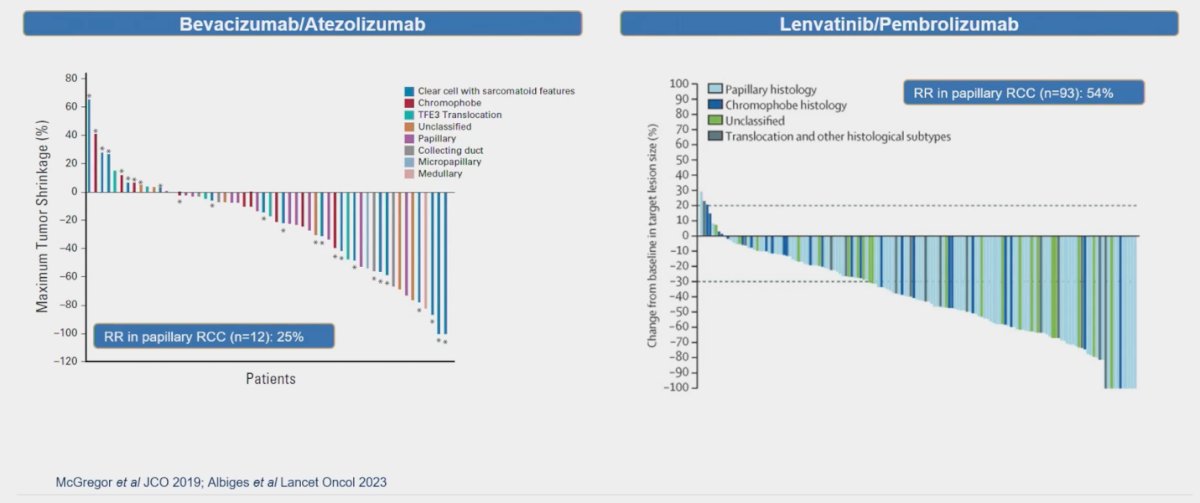
When specifically addressing patients with pRCC and MET-driven disease, Choueiri investigated the potential response to savolitinib, a receptor tyrosine kinase inhibitor targeting mesenchymal-epithelial transition factor (MET). In MET-driven pRCC, the response rate (RR) was 18%, while, as expected, patients with MET-independent pRCC showed no response.6 Additionally, in The CALYPSO study combining savolitinib with durvalumab for MET-driven pRCC, the RR was an impressive 53%.7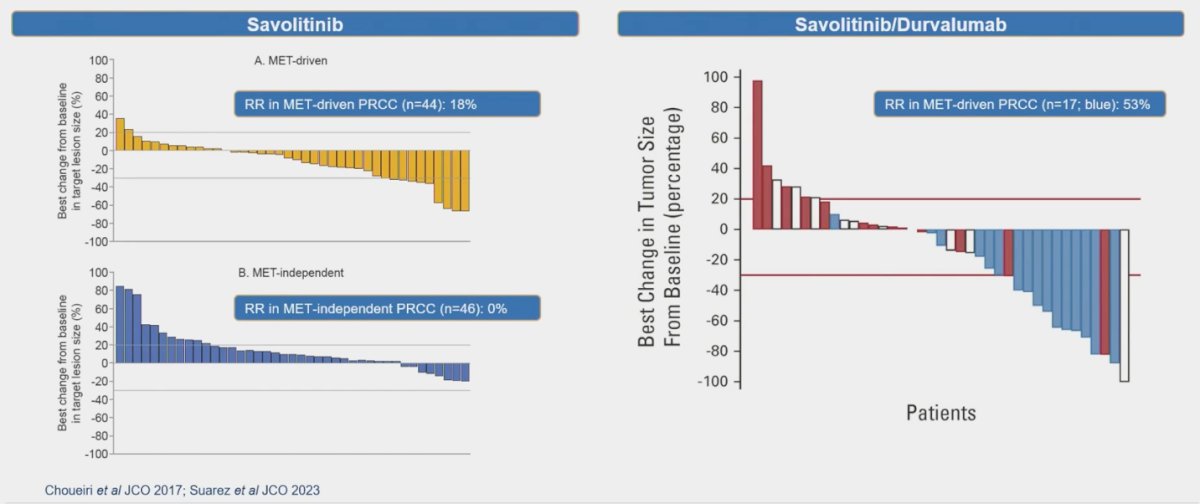
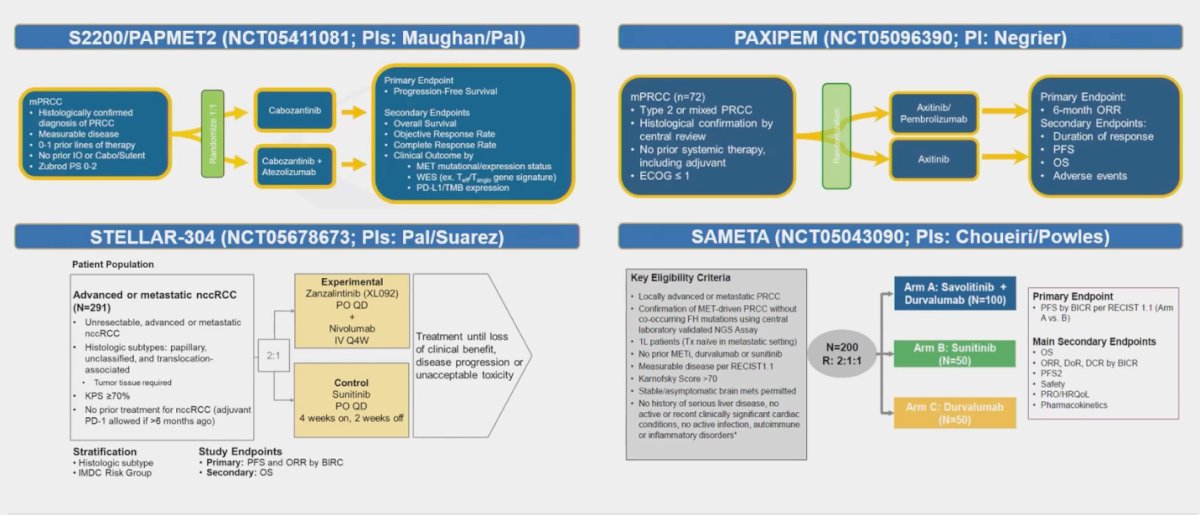
Dr. Kumar Pal highlighted four ongoing trials in pRCC, underscoring key aspects such as their phases, control arms, sample sizes, and study locations:
- S2200/PAPMET2 (NCT05411081): This randomized phase II study, led by Maughan and Pal, involves Cabozantinib as the control arm, with a sample size of 200 participants. The study location is in the United States.
- РАХІРЕМ (NCT05096390): Another randomized phase II trial, this study employs Axitinib as the control arm with a sample size of 72 participants. It is being conducted in France.
- STELLAR-304 (NCT05678673): This trial is a randomized phase III study with Sunitinib as the control arm, involving 291 participants. The study is being conducted at international locations.
- SAMETA (NCT05043090): Another randomized phase III trial, SAMETA, uses Sunitinib as the control arm with a sample size of 200 participants. The study is conducted at various international locations.
The current standard of care for pRCC was addressed by Dr. Kumar Pal, who suggested that Cabozantinib stands out as a prominent option. However, upon reviewing the European Association of Urology (EAU) RCC guidelines, both Cabozantinib and sunitinib receive only a weak recommendation. This underscores the need for positive RCT phase 3 trials to establish clearer guidelines for treating this variant histology.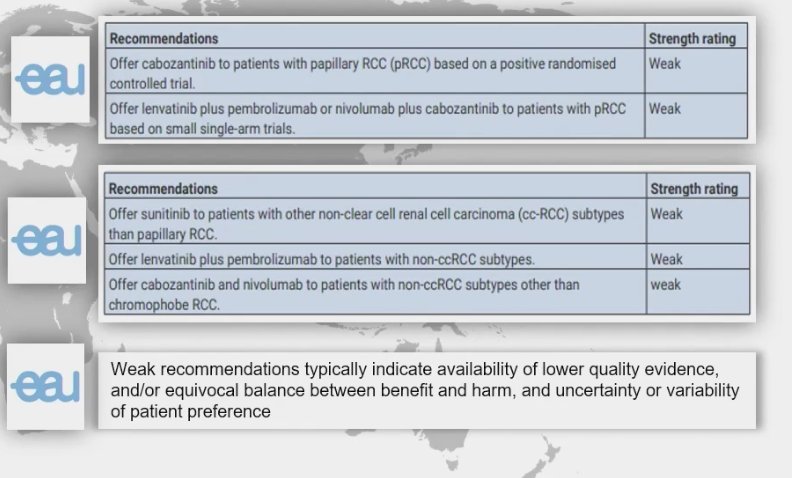
Chromophobe RCC (chRCC)
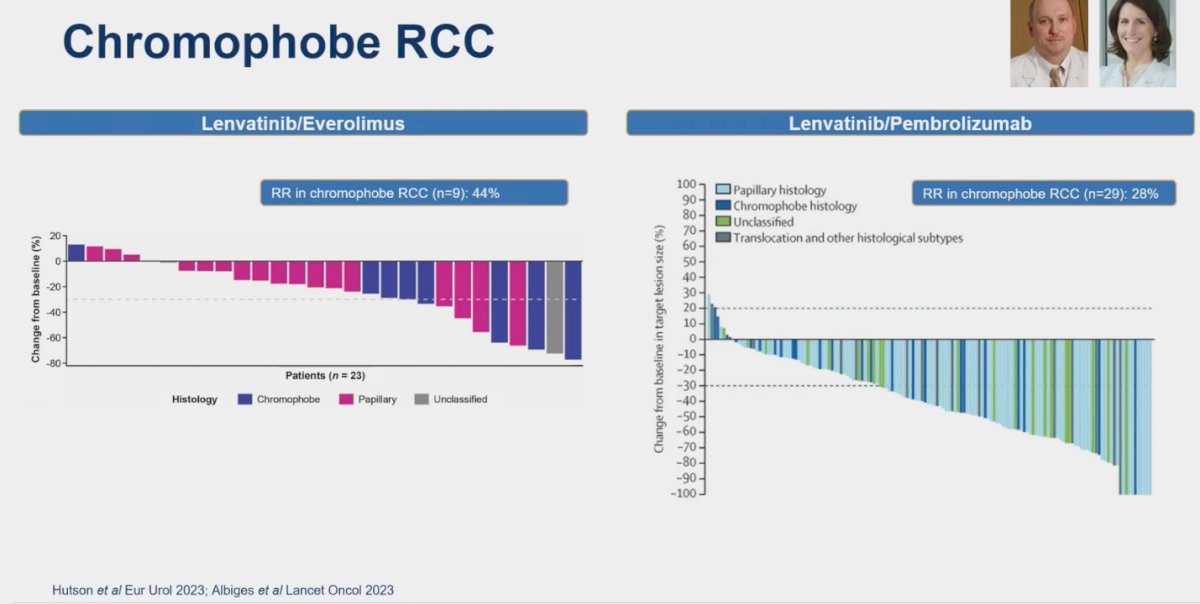
For chRCC, there is a notable lack of randomized controlled trials, with only single-arm studies available. Lenvatinib has emerged as a key player in the treatment landscape for this non-clear cell RCC histology. In one study, the combination of Lenvatinib and everolimus was investigated in a small cohort of patients (n=23), resulting in a response rate (RR) of 44%. Similarly, the combination of Lenvatinib and pembrolizumab was evaluated in 29 patients with chRCC, yielding a RR of 28% in this subgroup. Dr. Kumar Pal emphasized that while these response rates are not as high as anticipated, Lenvatinib represents one of the best treatment options available for chRCC based on current evidence.5
Collecting duct carcinoma
Collecting duct carcinoma (CDC) is a rare but highly aggressive type of renal cell carcinoma (RCC), accounting for 1%-2% of all RCC cases. CDC originates from the distal renal tubular epithelium, bearing a closer resemblance to urinary epithelial cells than renal cells. Treatment options for this disease are limited. In a prospective multicenter phase II study of gemcitabine plus platinum-based chemotherapy for metastatic CDC, 23 patients were assessed, yielding a response rate (RR) of 26% and a median progression-free survival (PFS) of 7 months.8 In a more contemporary study, Cabozantinib was explored for the treatment of CDC. This study also included 23 patients and reported a median PFS of 4 months and an RR of 35%.9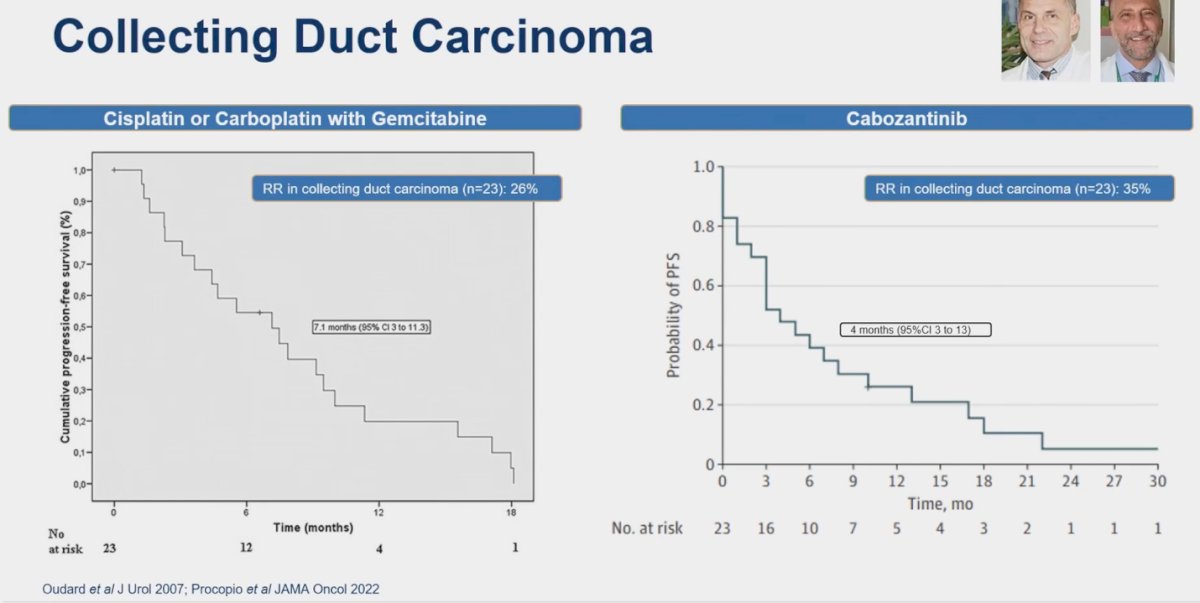
Renal medullary carcinoma
Tremendous efforts have been made to improve the treatment of this very rare non-clear cell RCC. A retrospective study analyzed the records of patients with renal medullary carcinoma (RMC) treated with gemcitabine plus doxorubicin. Among the 16 patients, three (18.8%) achieved a partial response, and seven (43.8%) achieved stable disease. The median progression-free survival (PFS) was 2.8 months, while the median overall survival (OS) was 8.1 months from the start of treatment.10
Dr. Kumar Pal presented his preferred treatment algorithm for renal medullary carcinoma, which is illustrated in the figure below. This algorithm reflects his approach to managing this aggressive and rare type of renal cell carcinoma, taking into account current evidence and clinical experience to optimize patient outcomes.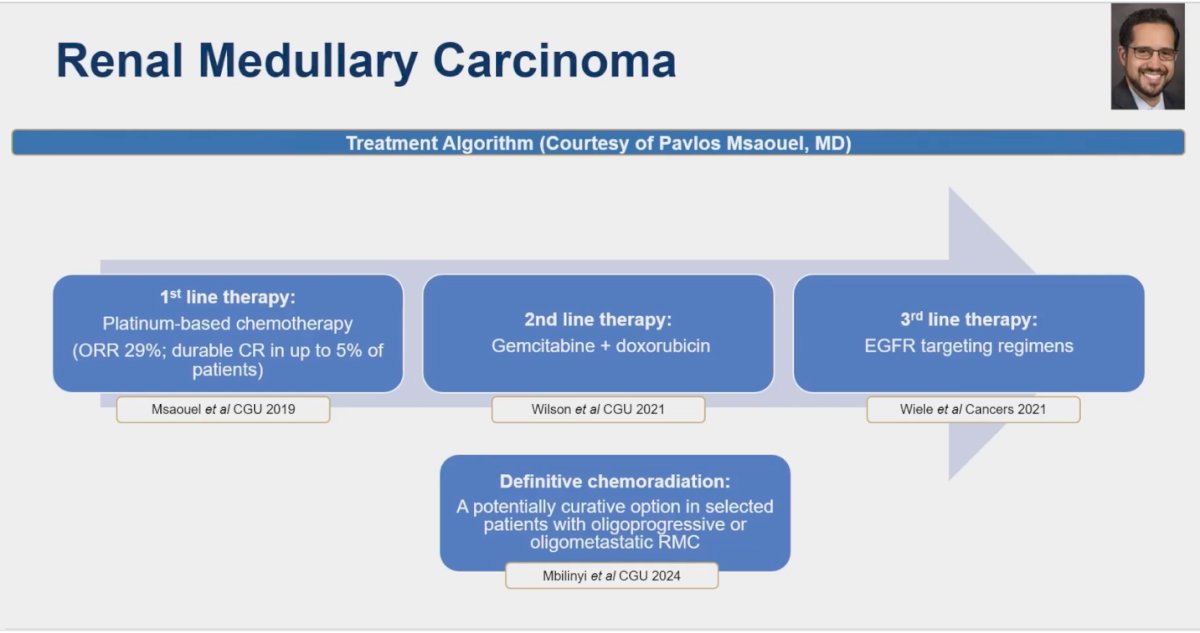
Hereditary Leiomyomatosis Renal cell carcinoma (HLRCC)
There is very limited data for the treatment of this rare non-clear cell RCC. Srinivasan explored the role of TKI + anti-VEGF therapy (Bevacizumab + Erlotinib) in this context. This study, the only prospective data available, analyzed 43 patients with HLRCC and reported a response rate (RR) of 72%. Notably, the RR in sporadic pRCC is almost half, at 35%. On the right side of the slide, there is an ongoing study testing the combination of bevacizumab, erlotinib, and atezolizumab in patients with HLRCC.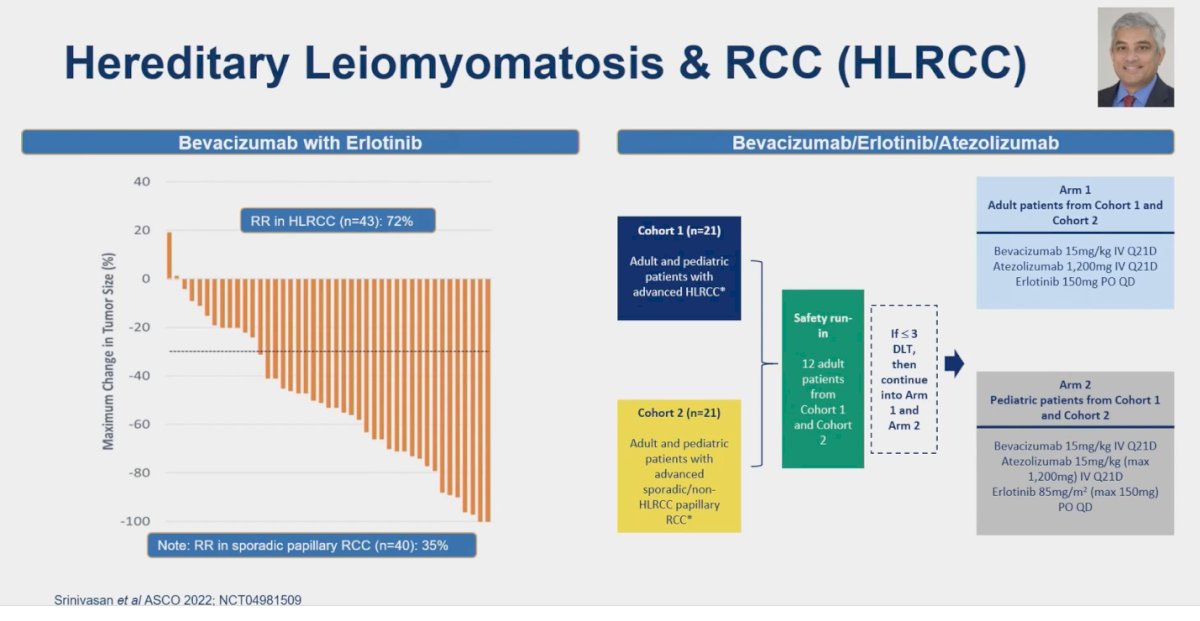
Translocation RCC
Translocation renal cell carcinoma (tRCC) is a rare variant of non-clear cell renal cell carcinoma characterized by gene fusions involving TFE3 and TFEB. Translocation RCC constitutes 1% to 5% of adult RCC but is more prevalent in childhood RCC. Data on systemic therapy for tRCC are limited and mainly derived from retrospective studies. In a study pooling retrospective data from 11 centers (n=29 patients), comparing treatment with IO/IO vs IO/TKI, the median PFS was 2.8 and 5.4 months in IO/IO and IO/TKI treatment groups, and the median OS from metastatic disease was 17.8 and 30.7 months, respectively. The treatment of translocation RCC with IO/IO compared to TKI + IO may be associated with a limited response and increased progressive disease as outlined in the figure below.11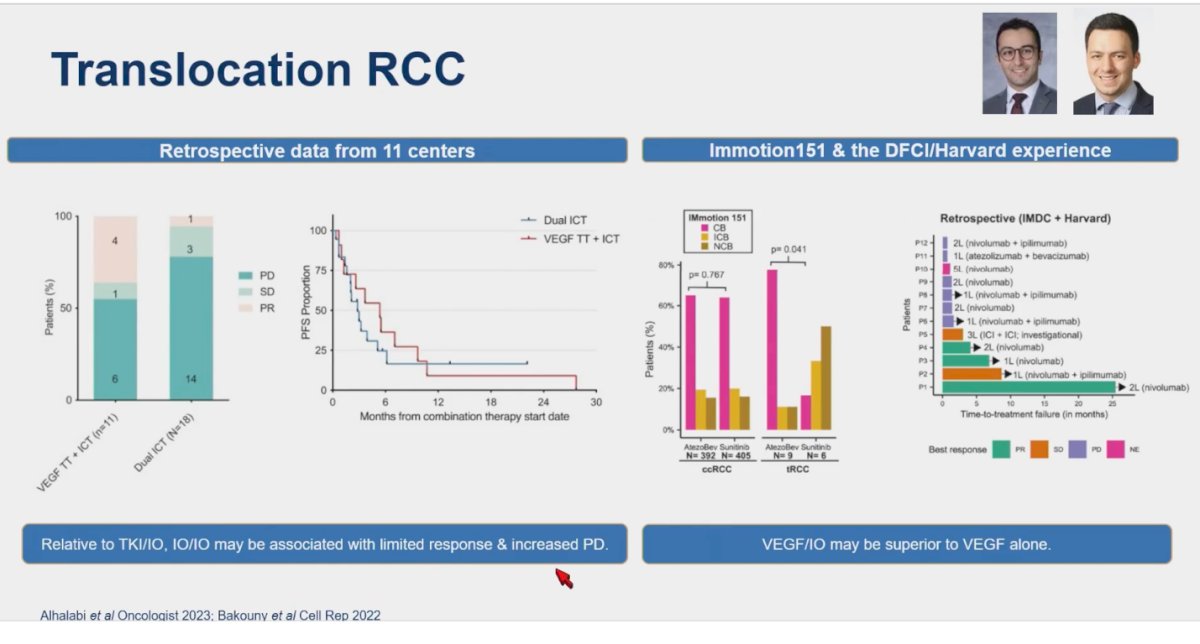
Similarly, in an analysis of the Immotion 151 trial and the DFCI/Harvard experience, including 15 patients with tRCC, VEGF/IO combinations showed superior outcomes compared to sunitinib alone. The Bevacizumab and Atezolizumab combination may help address this treatment conundrum, but it is important to note that this conclusion is based on retrospective data from Harvard and the Immotion randomized clinical trial, which were not specifically powered for tRCC.
Dr. Kumar Pal discussed methods to find clinical trials for patients with non-clear cell RCC, emphasizing the utility of the Kidney Cure website. He highlighted that this platform offers a well-curated list of clinical trials specifically targeting patients with these rare variants, making it a valuable resource for both clinicians and patients seeking cutting-edge treatment options.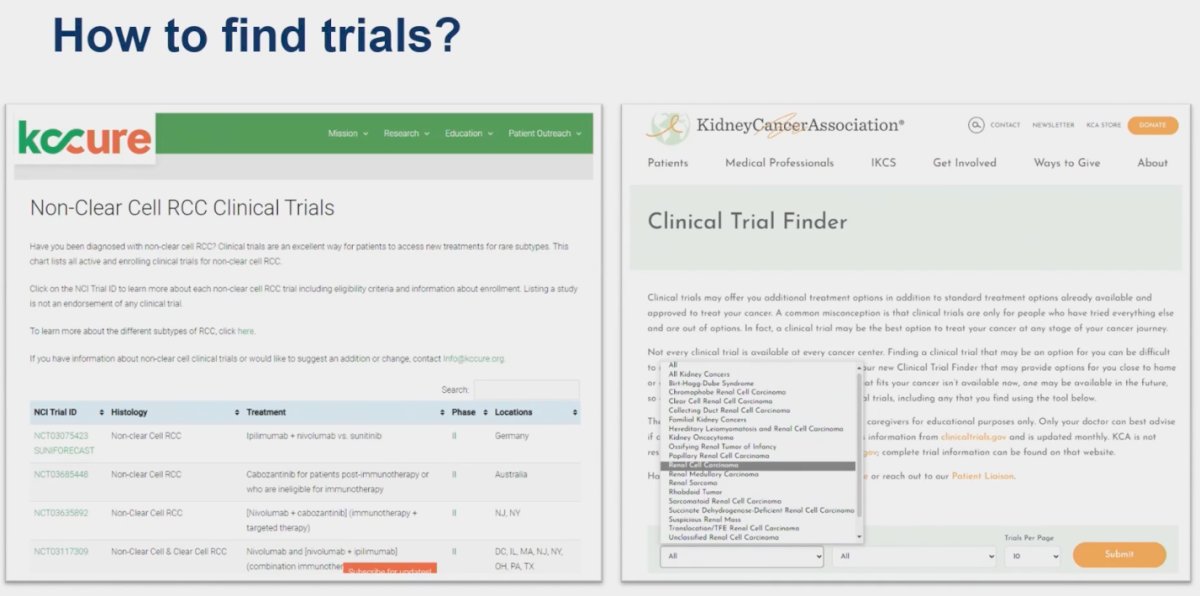
Lastly, the nomenclature of these cancers has been brought into discussion recently, raising questions about whether the term "non-clear cell RCC" should be changed. Some advocate for calling them "rare variant kidney cancers." However, a recent Twitter poll concluded that we should maintain the term "non-clear cell" while working towards ensuring patients can identify treatment options and trials for specific non-clear cell subtypes. Additionally, a poll on the Kidney Cure website, with over 180 votes, reached a consensus to maintain the "non-clear cell RCC" name.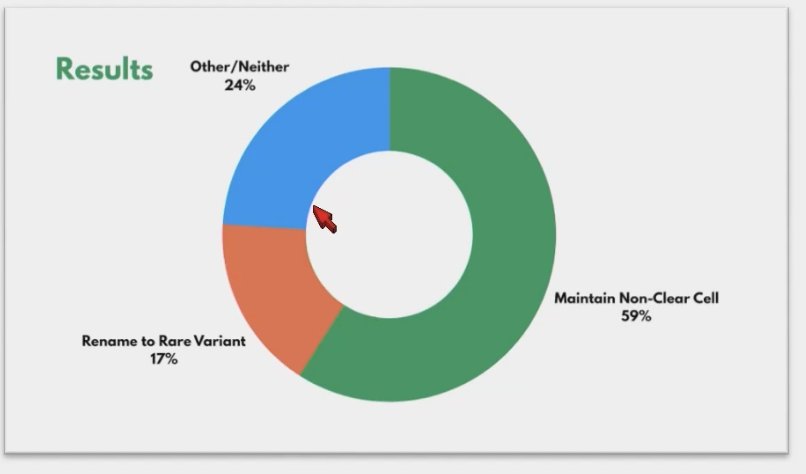
Dr. Kumar Pal wrapped his presentation with the following key messages:
- There have been randomized phase Il trials in papillary RCC, but optimal therapy is still debatable
- Single-arm studies in exquisitely rare histologies have been successfully conducted (collecting duct, renal medullary cancer) and can guide therapy
- Multiple randomized studies are ongoing; tools exist to guide patients towards the trials & honing existing nomenclature may help
Presented by: Sumanta Kumar Pal, MD, FASCO, Professor, Department of Medical Oncology & Therapeutics Research, City of Hope Comprehensive Cancer Center, Los Angeles, CA
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31st and June 4th.
References:
- Pal SK, Tangen C, Thompson IM Jr, Balzer-Haas N, George DJ, Heng DYC, Shuch B, Stein M, Tretiakova M, Humphrey P, Adeniran A, Narayan V, Bjarnason GA, Vaishampayan U, Alva A, Zhang T, Cole S, Plets M, Wright J, Lara PN Jr. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: a randomised, open-label, phase 2 trial. Lancet. 2021 Feb 20;397(10275):695-703. doi: 10.1016/S0140-6736(21)00152-5. Epub 2021 Feb 13. PMID: 33592176; PMCID: PMC8687736.
- Pal SK, McGregor B, Suárez C, Tsao CK, Kelly W, Vaishampayan U, Pagliaro L, Maughan BL, Loriot Y, Castellano D, Srinivas S, McKay RR, Dreicer R, Hutson T, Dubey S, Werneke S, Panneerselvam A, Curran D, Scheffold C, Choueiri TK, Agarwal N. Cabozantinib in Combination With Atezolizumab for Advanced Renal Cell Carcinoma: Results From the COSMIC-021 Study. J Clin Oncol. 2021 Nov 20;39(33):3725-3736. doi: 10.1200/JCO.21.00939. Epub 2021 Sep 7. PMID: 34491815; PMCID: PMC8601305.
- Fitzgerald KN, Lee CH, Voss MH, Carlo MI, Knezevic A, Peralta L, Chen Y, Lefkowitz RA, Shah NJ, Owens CN, McHugh DJ, Aggen DH, Laccetti AL, Kotecha RR, Feldman DR, Motzer RJ. Cabozantinib Plus Nivolumab in Patients with Non-Clear Cell Renal Cell Carcinoma: Updated Results from a Phase 2 Trial. Eur Urol. 2024 May 22:S0302-2838(24)02349-2. doi: 10.1016/j.eururo.2024.04.025. Epub ahead of print. PMID: 38782695.
- McGregor BA, McKay RR, Braun DA, Werner L, Gray K, Flaifel A, Signoretti S, Hirsch MS, Steinharter JA, Bakouny Z, Flippot R, Wei XX, Choudhury A, Kilbridge K, Freeman GJ, Van Allen EM, Harshman LC, McDermott DF, Vaishampayan U, Choueiri TK. Results of a Multicenter Phase II Study of Atezolizumab and Bevacizumab for Patients With Metastatic Renal Cell Carcinoma With Variant Histology and/or Sarcomatoid Features. J Clin Oncol. 2020 Jan 1;38(1):63-70. doi: 10.1200/JCO.19.01882. Epub 2019 Nov 13. PMID: 31721643; PMCID: PMC7051851.
- Albiges L, Gurney H, Atduev V, Suarez C, Climent MA, Pook D, Tomczak P, Barthelemy P, Lee JL, Stus V, Ferguson T, Wiechno P, Gokmen E, Lacombe L, Gedye C, Perini RF, Sharma M, Peng X, Lee CH. Pembrolizumab plus lenvatinib as first-line therapy for advanced non-clear-cell renal cell carcinoma (KEYNOTE-B61): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2023 Aug;24(8):881-891. doi: 10.1016/S1470-2045(23)00276-0. Epub 2023 Jul 11. PMID: 37451291.
- Choueiri TK, Plimack E, Arkenau HT, Jonasch E, Heng DYC, Powles T, Frigault MM, Clark EA, Handzel AA, Gardner H, Morgan S, Albiges L, Pal SK. Biomarker-Based Phase II Trial of Savolitinib in Patients With Advanced Papillary Renal Cell Cancer. J Clin Oncol. 2017 Sep 10;35(26):2993-3001. doi: 10.1200/JCO.2017.72.2967. Epub 2017 Jun 23. PMID: 28644771.
- Suárez C, Larkin JMG, Patel P, Valderrama BP, Rodriguez-Vida A, Glen H, Thistlethwaite F, Ralph C, Srinivasan G, Mendez-Vidal MJ, Hartmaier R, Markovets A, Prendergast A, Szabados B, Mousa K, Powles T. Phase II Study Investigating the Safety and Efficacy of Savolitinib and Durvalumab in Metastatic Papillary Renal Cancer (CALYPSO). J Clin Oncol. 2023 May 10;41(14):2493-2502. doi: 10.1200/JCO.22.01414. Epub 2023 Feb 21. Erratum in: J Clin Oncol. 2023 Aug 10;41(23):3961. PMID: 36809050.
- Oudard S, Banu E, Vieillefond A, Fournier L, Priou F, Medioni J, Banu A, Duclos B, Rolland F, Escudier B, Arakelyan N, Culine S; GETUG (Groupe d'Etudes des Tumeurs Uro-Génitales). Prospective multicenter phase II study of gemcitabine plus platinum salt for metastatic collecting duct carcinoma: results of a GETUG (Groupe d'Etudes des Tumeurs Uro-Génitales) study. J Urol. 2007 May;177(5):1698-702. doi: 10.1016/j.juro.2007.01.063. PMID: 17437788.
- Procopio G, Sepe P, Claps M, Buti S, Colecchia M, Giannatempo P, Guadalupi V, Mariani L, Lalli L, Fucà G, de Braud F, Verzoni E. Cabozantinib as First-line Treatment in Patients With Metastatic Collecting Duct Renal Cell Carcinoma: Results of the BONSAI Trial for the Italian Network for Research in Urologic-Oncology (Meet-URO 2 Study). JAMA Oncol. 2022 Jun 1;8(6):910-913. doi: 10.1001/jamaoncol.2022.0238. PMID: 35420628; PMCID: PMC9011175.
- Wilson NR, Wiele AJ, Surasi DS, Rao P, Sircar K, Tamboli P, Shah AY, Genovese G, Karam JA, Wood CG, Tannir NM, Msaouel P. Efficacy and safety of gemcitabine plus doxorubicin in patients with renal medullary carcinoma. Clin Genitourin Cancer. 2021 Dec;19(6):e401-e408. doi: 10.1016/j.clgc.2021.08.007. Epub 2021 Sep 15. PMID: 34625389.
- Alhalabi O, Thouvenin J, Négrier S, Vano YA, Campedel L, Hasanov E, Bakouny Z, Hahn AW, Bilen MA, Msaouel P, Choueiri TK, Viswanathan SR, Sircar K, Albiges L, Malouf GG, Tannir NM. Immune Checkpoint Therapy Combinations in Adult Advanced MiT Family Translocation Renal Cell Carcinomas. Oncologist. 2023 May 8;28(5):433-439. doi: 10.1093/oncolo/oyac262. PMID: 36640141; PMCID: PMC10166175.
- Bakouny Z, Sadagopan A, Ravi P, Metaferia NY, Li J, AbuHammad S, Tang S, Denize T, Garner ER, Gao X, Braun DA, Hirsch L, Steinharter JA, Bouchard G, Walton E, West D, Labaki C, Dudani S, Gan CL, Sethunath V, Carvalho FLF, Imamovic A, Ricker C, Vokes NI, Nyman J, Berchuck JE, Park J, Hirsch MS, Haq R, Mary Lee GS, McGregor BA, Chang SL, Feldman AS, Wu CJ, McDermott DF, Heng DYC, Signoretti S, Van Allen EM, Choueiri TK, Viswanathan SR. Integrative clinical and molecular characterization of translocation renal cell carcinoma. Cell Rep. 2022 Jan 4;38(1):110190. doi: 10.1016/j.celrep.2021.110190. PMID: 34986355; PMCID: PMC9127595.


