(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31 and June 4 was host to the session Advancing Prostate Cancer Care: Treatment Approaches to Precision Medicine, Biomarker Innovations, and Equitable Access. Dr. Delphine Chen discussed the role of molecular imaging in prostate cancer and when and which agents we should be using.
Dr. Chen began by explaining that molecular imaging is now integral to prostate cancer (PCa) care, aiding in risk assessment, treatment decisions, prognosis, and follow-up. The advent of ligands capable of imaging prostate-specific membrane antigen (PSMA) via positron emission tomography (PET) has revolutionized PCa imaging. It has brought nuclear medicine into a central role in the care of PCa patients. Frequently imaging modalities used for PCa staging and diagnosis are magnetic resonance imaging (MRI), computerized tomography (CT) scans, and bone scans. PET-PSMA has opened our eyes in terms of what we can see in regard of where PCa can be seen specifically in the early stages of the disease (initial staging, biochemical recurrence).
The FDA has approved several PSMA agents in the United States. Starting in 2020 with Ga-68 PSMA-11 (USCF/UCLA, kit approvals 2021 and 2022), in 2021 F-18 piflufolastat/DCFPyL, in 2022 Lu-177 vipivotide tetraxetan and 2023 F-18 flotufolastat/rhPSMA-7.3.
Multiple applications for PSMA-PET have been proposed before, but briefly, they can be grouped into three major settings:
- Initial staging
- Biochemically recurrent PCa
- Selection for PSMA-Targeted Radioligand Therapy
PSMA-PET for initial prostate cancer (PCa) staging has been extensively studied in multiple prospective clinical trials. Dr. Chen discussed the most significant trials in this context. Most trials included men with high-risk prostate adenocarcinoma, defined as clinical stage ≥T3a or a PSA level >20 ng/mL, or a Gleason score ≥8.1-4 The ProPSMA trial also encompassed patients with ISUP grade group 3 disease(2), while the LIGHTHOUSE trial, besides men with high-risk disease, also enrolled patients with unfavorable intermediate-risk PCa: GG3 or GG 2 with ≥50% of biopsy cores positive for PCa and/or >1 intermediate-risk factor (T2b; T2c; PSA level 10-20 ng/mL).3
The ProPSMA trial demonstrated that PSMA-PET, compared to conventional imaging (CT and bone scan) in patients undergoing radical prostatectomy, was more sensitive in detecting metastatic disease in all sites (pelvic nodal and distant metastasis), and it was highly specific.2
Besides this, all the aforementioned trials demonstrated higher sensitivity, specificity, and accuracy compared with conventional imaging. However, when comparing the diagnostic performance in detecting nodal metastases in high-risk disease across all trials, all agents exhibited limited sensitivities (27-40%) except for the ProPSMA trial (Table below). Interestingly, when reviewing the post hoc analysis of all these trials, the ability to identify lymph nodes decreases with smaller nodes (<1cm), as PET can have resolution issues, particularly when there is low uptake. However, it is worth noting that the specificity to detect pelvic nodal metastasis was very high across all studies, ranging from 95% to 98%.1-4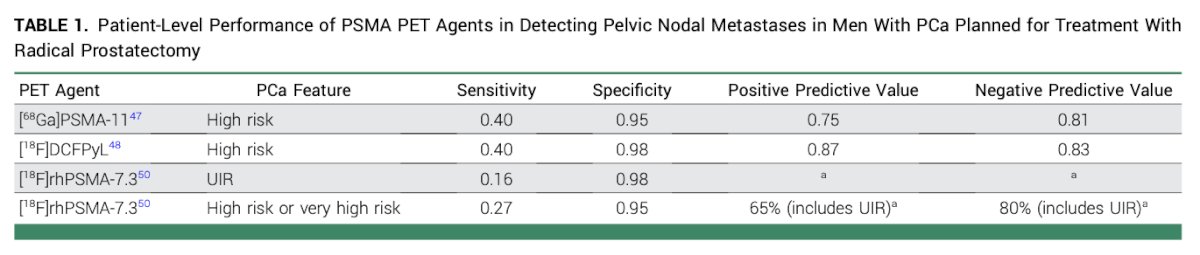
Increasingly, we are observing a larger number of patients with PSMA PET-positive mesorectal nodes that were not identified using conventional imaging. When these nodal metastases are treated with radiation therapy (RT), PSA levels decline in patients with biochemical recurrence.
Biochemically recurrent (BCR) prostate cancer (PCa) is likely the setting where most initial studies evaluating the performance of PSMA PET in detecting PCa were conducted. Dr. Chen discussed three studies in this setting: the UCLA/UCSF-led trial, CONDOR, and SPOTLIGHT trials evaluating [68Ga]PSMA-11, F-18 DCFPyL, and F-18 rhPSMA, respectively.5-7
In patients with BCR, conventional imaging, particularly bone scans, relies on a high PSA level for positivity. For bone scans, the PSA should be above 20 to indicate a significant likelihood of positivity, rendering the bone scan very insensitive to BCR disease. Dr. Chen argued that despite the lower detection rate when the PSA level is <0.5 ng/mL, the 36%-44% detection rate is still higher than that observed with conventional imaging. The results of three studies assessing PSMA PET, stratified by PSA level in BCR, are summarized in the table below:
Finally, another crucial role of PSMA-PET is in selecting patients for PSMA-Targeted Radioligand Therapy. Dr. Chen discussed how the field of theranostics has significantly expanded since the FDA approval of [177Lu]PSMA-617 (Lu-177 vipivotide tetraxetan, Pluvicto).
Theranostics implies, labeling a drug with an imaging radioisotope that can be then swapped for a therapy isotope. Another indication for PSMA PET is for selecting patients for PSMA-targeted radioligand therapy.
The VISION trial was a multicentre, open-label, randomised, phase 3 trial evaluating the efficacy of Lu-177 PSMA-617 in patients with metastatic castration-resistant prostate cancer (mCRPC) who have received previous androgen receptor pathway inhibitor and taxane treatment. In the VISION study, Lu-177 PSMA-617 significantly delayed time to first symptomatic skeletal event, with median time to event of 11.5 months in the treatment group compared to 6.8 months in the control group.8
The TheraP trial was a randomized, two-arm multicentre phase II trial in men with mCRPC post docetaxel who were suitable for cabazitaxel.9 This trial compared the efficacy of Lu-177 PSMA-617 with cabazitaxel. The TheraP trial showed that overall survival did not significantly differ between participants assigned to Lu-177 PSMA-617 and those assigned to cabazitaxel (median 19.1 months vs. 19.6 months, p=0.77).
The eligibility criteria for Lu-177 PSMA-617 varied between the VISION and TheraP trials. In brief, the main differences were that in TheraP, all lesions had to have a maximum SUV > 10, and in some cases, FDG-PET was used (discordance with PSMA PET was not allowed).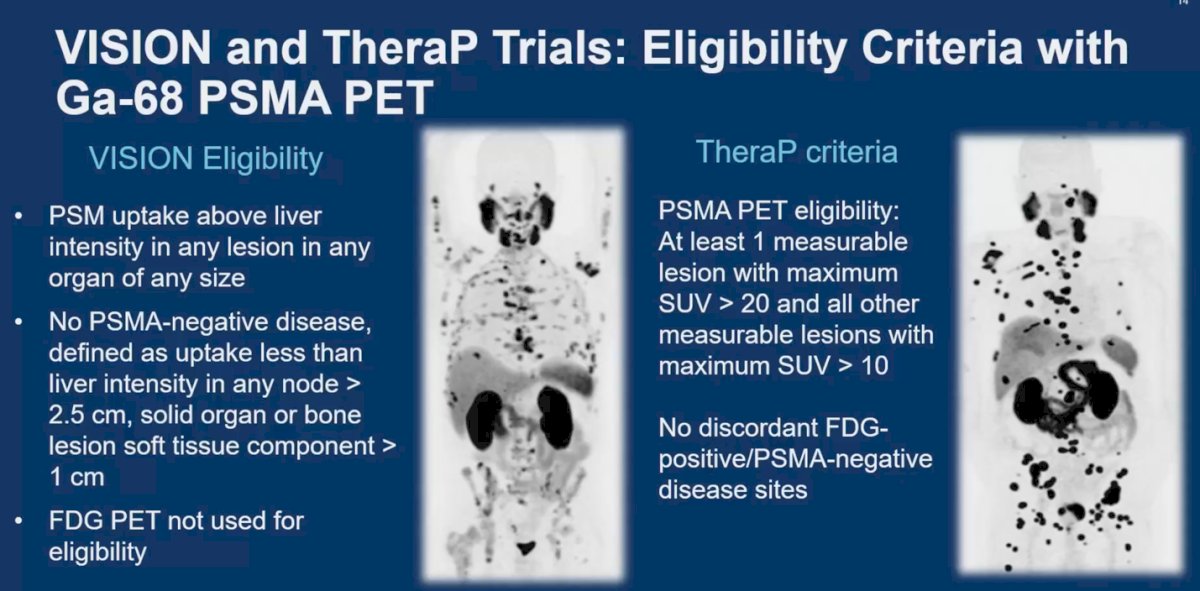
Dr. Chen discussed that in clinical practice, we don’t have to strictly adhere to the eligibility criteria used in clinical trials. However, for patient selection and treatment eligibility for Lu-177 PSMA, we should consider the eligibility criteria from the trials, along with other factors such as comorbidities, performance status, and the patient's ability to tolerate treatment with Lu-177 PSMA-617 to guide our treatment decision-making. She highlighted three crucial factors predicting response to Lu-177 PSMA-617:
- Highly PSMA expression: Predicts a good response.
- Intermediate to low PSMA expression with discordant disease on FDG PET: Predicts an intermediate response.
- Highly discordant disease on FDG PET: Predicts a poor response. Normally, treatment with PSMA would not be recommended in these cases
Finally, Dr. Chen delved into radiopharmacy networks and distribution areas in the US, considering the isotope half-life. Ga-68, with a half-life of 68 minutes, necessitates radiopharmacies to be in close proximity to where the PET PSMA scans are conducted. The graphic below illustrates the distribution of both Ga-68 and F-18 radiopharmaceuticals networks. Remarkably, some regions in the US are not conveniently situated concerning radiopharmacy access.
Furthermore, other factors impacting access to PSMA PET include local cyclotron capacity for F-18 production, as well as the operational windows available for F-18 production. Some cyclotrons also produce FDG and other F-18 radiopharmaceuticals, which can affect scheduling. Additionally, insurance reimbursement for molecular imaging studies represents another limiting factor.
Dr Chen concluded her presentation delivering the following key messages:
- PSMA PET has high clinical utility in initial staging, detection of biochemically recurrent prostate cancer, and patient selection for PSMA radioligand therapy
- Criteria for determining PSMA therapy eligibility have been described in clinical trial, but optimal criteria are still evolving
- Being able to treat patients with PSMA radioligand therapy is a major advancement for nuclear medicine and for patients with PCa. We can further refine the use of PSMA and FDG PET to improve patient outcomes.
- Access to PSMA PET is improving with increased distribution networks; however, cost and reimbursement are barriers that we would have to overcome.
Presented by: Delphine L. Chen, MD, Professor in Nuclear Medicine and Director of Molecular Imaging at the Fred Hutchinson Cancer Center
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31st and June 4th.
References:
- Hope TA, Eiber M, Armstrong WR, Juarez R, Murthy V, Lawhn-Heath C, Behr SC, Zhang L, Barbato F, Ceci F, Farolfi A, Schwarzenböck SM, Unterrainer M, Zacho HD, Nguyen HG, Cooperberg MR, Carroll PR, Reiter RE, Holden S, Herrmann K, Zhu S, Fendler WP, Czernin J, Calais J. Diagnostic Accuracy of 68Ga-PSMA-11 PET for Pelvic Nodal Metastasis Detection Prior to Radical Prostatectomy and Pelvic Lymph Node Dissection: A Multicenter Prospective Phase 3 Imaging Trial. JAMA Oncol. 2021 Nov 1;7(11):1635-1642 doi: 10.1001/jamaoncol.2021.3771. PMID: 34529005; PMCID: PMC8446902. .
- Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, Rutherford N, Martin JM, Frydenberg M, Shakher R, Wong LM, Taubman K, Ting Lee S, Hsiao E, Roach P, Nottage M, Kirkwood I, Hayne D, Link E, Marusic P, Matera A, Herschtal A, Iravani A, Hicks RJ, Williams S, Murphy DG; proPSMA Study Group Collaborators. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020 Apr 11;395(10231):1208-1216.. doi: 10.1016/S0140-6736(20)30314-7. Epub 2020 Mar 22. PMID: 32209449.
- Surasi DS, Eiber M, Maurer T, Preston MA, Helfand BT, Josephson D, Tewari AK, Somford DM, Rais-Bahrami S, Koontz BF, Bostrom PJ, Chau A, Davis P, Schuster DM, Chapin BF; LIGHTHOUSE Study Group. Diagnostic Performance and Safety of Positron Emission Tomography with 18F-rhPSMA-7.3 in Patients with Newly Diagnosed Unfavourable Intermediate- to Very-high-risk Prostate Cancer: Results from a Phase 3, Prospective, Multicentre Study (LIGHTHOUSE). Eur Urol. 2023 Oct;84(4):361-370.
- Pienta KJ, Gorin MA, Rowe SP, Carroll PR, Pouliot F, Probst S, Saperstein L, Preston MA, Alva AS, Patnaik A, Durack JC, Stambler N, Lin T, Jensen J, Wong V, Siegel BA, Morris MJ. A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with 18F-DCFPyL in Prostate Cancer Patients (OSPREY). J Urol. 2021 Jul;206(1):52-61. doi: 10.1097/JU.0000000000001698. Epub 2021 Feb 26. PMID: 33634707; PMCID: PMC8556578.
- Fendler WP, Calais J, Eiber M, Flavell RR, Mishoe A, Feng FY, Nguyen HG, Reiter RE, Rettig MB, Okamoto S, Emmett L, Zacho HD, Ilhan H, Wetter A, Rischpler C, Schoder H, Burger IA, Gartmann J, Smith R, Small EJ, Slavik R, Carroll PR, Herrmann K, Czernin J, Hope TA. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol. 2019 Jun 1;5(6):856-863.doi: 10.1001/jamaoncol.2019.0096. PMID: 30920593; PMCID: PMC6567829.
- Morris MJ, Rowe SP, Gorin MA, Saperstein L, Pouliot F, Josephson D, Wong JYC, Pantel AR, Cho SY, Gage KL, Piert M, Iagaru A, Pollard JH, Wong V, Jensen J, Lin T, Stambler N, Carroll PR, Siegel BA; CONDOR Study Group. Diagnostic Performance of 18F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase III, Multicenter Study. Clin Cancer Res. 2021 Jul 1;27(13):3674-3682. doi: 10.1158/1078-0432.CCR-20-4573. Epub 2021 Feb 23. PMID: 33622706; PMCID: PMC8382991.
- Jani AB, Ravizzini GC, Gartrell BA, Siegel BA, Twardowski P, Saltzstein D, Fleming MT, Chau A, Davis P, Chapin BF, Schuster DM; SPOTLIGHT Study Group. Diagnostic Performance and Safety of 18F-rhPSMA-7.3 Positron Emission Tomography in Men With Suspected Prostate Cancer Recurrence: Results From a Phase 3, Prospective, Multicenter Study (SPOTLIGHT). J Urol. 2023 Aug;210(2):299-311. doi: 10.1097/JU.0000000000003493. Epub 2023 Apr 26. PMID: 37126069.
- Fizazi K, Herrmann K, Krause BJ, Rahbar K, Chi KN, Morris MJ, Sartor O, Tagawa ST, Kendi AT, Vogelzang N, Calais J, Nagarajah J, Wei XX, Koshkin VS, Beauregard JM, Chang B, Ghouse R, DeSilvio M, Messmann RA, de Bono J. Health-related quality of life and pain outcomes with [177Lu]Lu-PSMA-617 plus standard of care versus standard of care in patients with metastatic castration-resistant prostate cancer (VISION): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2023 Jun;24(6):597-610. doi: 10.1016/S1470-2045(23)00158-4. PMID: 37269841; PMCID: PMC10641914.
- Hofman MS, Emmett L, Violet J, Y Zhang A, Lawrence NJ, Stockler M, Francis RJ, Iravani A, Williams S, Azad A, Martin A, McJannett M; ANZUP TheraP team; Davis ID. TheraP: a randomized phase 2 trial of 177Lu-PSMA-617 theranostic treatment vs cabazitaxel in progressive metastatic castration-resistant prostate cancer (Clinical Trial Protocol ANZUP 1603). BJU Int. 2019 Nov;124 Suppl 1:5-13. doi: 10.1111/bju.14876. Epub 2019 Oct 22. PMID: 31638341.


