(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured a session on prostate cancer, and a presentation by Dr. Matthew Smith discussing results of CYCLONE 2, a phase 3 trial assessing abemaciclib with abiraterone in patients with metastatic castration-resistant prostate cancer (mCRPC). Oncogenic addiction to androgen receptor signaling drives mCRPC progression, highlighting the unmet need for novel treatment strategies to maximize androgen receptor-directed therapy. Preclinical evidence suggests a key role for CDK4/6 in sustained androgen receptor signaling, uncontrolled proliferation, and hormonal resistance in prostate cancer. Abemaciclib is a potent CDK4/6 oral inhibitor that significantly augments the efficacy of endocrine therapy in hormonally driven (ER+) high-risk early-stage and metastatic breast cancer:
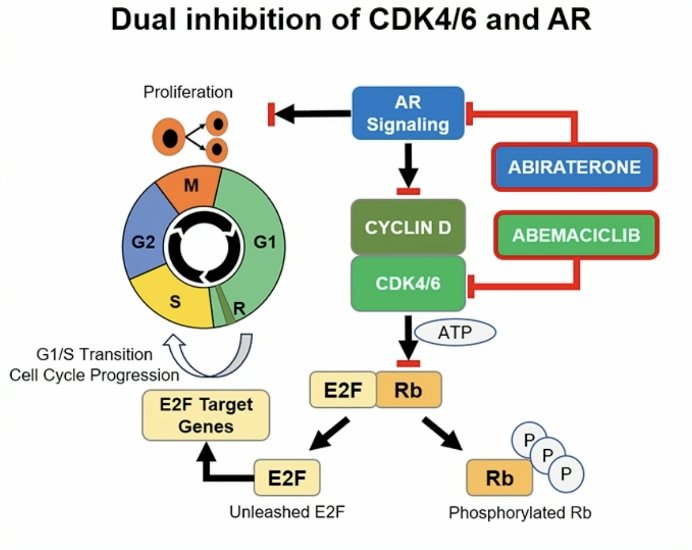
Moreover, abemaciclib also showed single-agent activity in heavily pretreated mCRPC (CYCLONE 1). At the 2024 ASCO annual meeting, Dr. Smith and colleagues report the primary results of CYCLONE 2, a phase 3 study of abemaciclib plus abiraterone in patients with first line mCRPC.
CYCLONE 2 was a seamless phase 2/3 adaptive trial with a dose-finding safety lead-in (n = 46). Part 2 (n = 146) was expanded given a radiologic progression free survival advantage of HR < 0.668, and part 3 (n = 201) included enrollment expansion. Key eligibility included mCRPC, visceral metastases were allowed (including liver metastases), ECOG performance status 0-1, and continuous ADT. Patients could not have prior treatment with CDK4/6 inhibitors, androgen receptor pathway inhibitors, or chemotherapy for mCRPC (prior docetaxel for mCSPC was permitted). Randomization to the abemaciclib or placebo plus abiraterone and predniso(lo)ne was stratified by prior docetaxel receipt for mHSPC, measurable disease, and radiographic progression at study entry. The trial design for CYCLONE 2 is as follows:
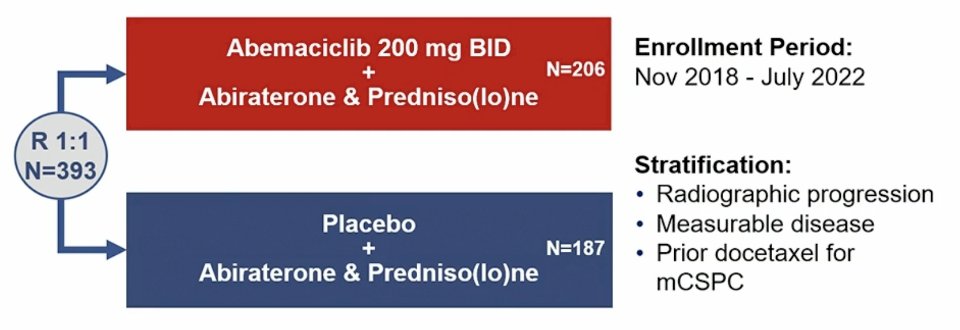
The primary endpoint was investigator-assessed radiographic progression-free survival per RECIST v1.1 and PCWG3 in the intention to treat population (Parts 1-3). Secondary endpoints included:
- Radiographic progression free survival by blinded independent central review
- Overall survival
- Objective response rate
- Duration of response
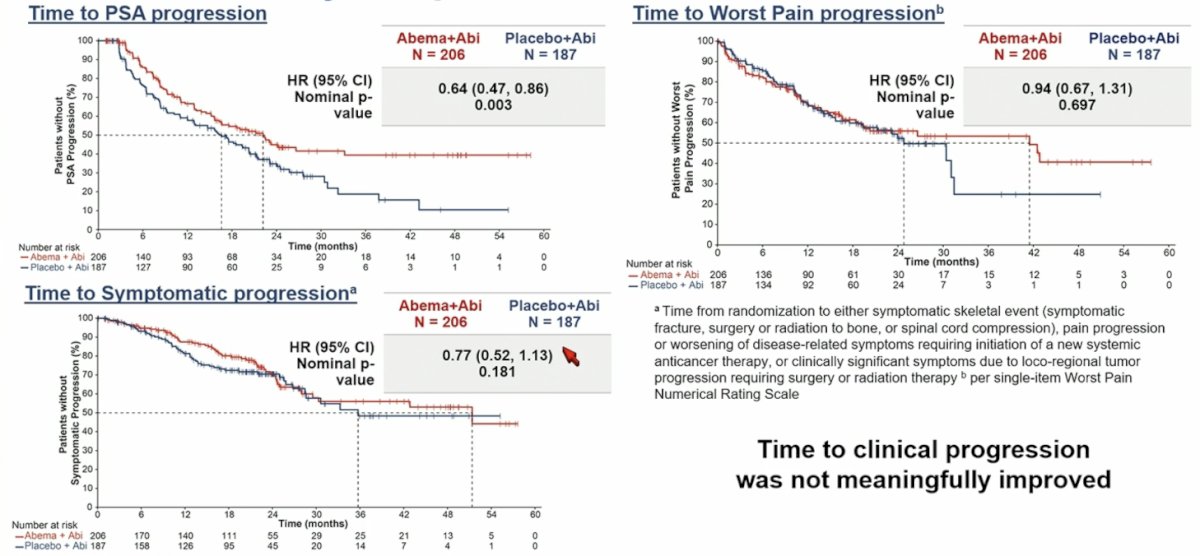
- Time to symptomatic progression
- Time to PSA progression
- Time to worst pain progression
- Safety
- Pharmacokinetics
The study was powered at ~90%, assuming a HR of 0.55 for radiographic progression-free survival, at a cumulative 2-sided alpha level of 0.05. Overall survival was a gated secondary endpoint were not controlled for Type 1 error.
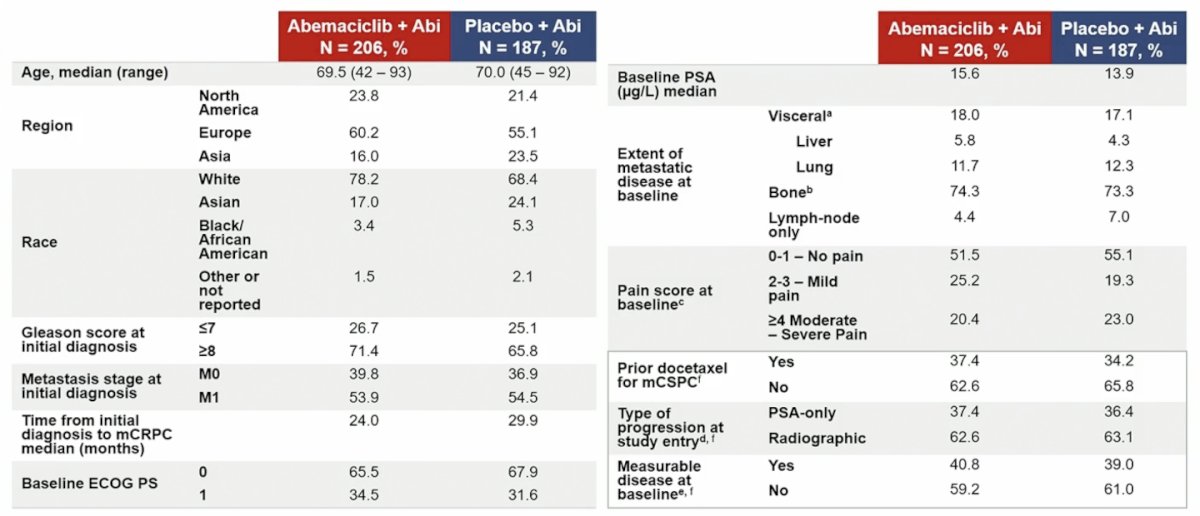
Between November 2018 and July 2022, 393 patients were randomized, with balanced baseline characteristics between arms:
In this trial, the primary endpoint of radiographic progression-free survival was not met (HR 0.83; 95% CI, 0.62–1.11; p = 0.212), with medians of 22.0 months for the abemaciclib plus abiraterone group versus 20.3 months for the placebo plus abiraterone group

Radiographic progression-free survival by blinded independent central review was consistent with the investigator assessment (HR 0.84; 95% CI, 0.61–1.16). Overall survival was a gated secondary endpoint and not inferentially tested (HR 0.93; 95% CI, 0.67–1.29; 38.9% maturity):

The objective response rate for the abemaciclib plus abiraterone group was 45% and was 55% for placebo plus abiraterone. Confirmed PSA response rate is as follows:

Other secondary endpoints included time to PSA progression (HR 0.64; 95% CI, 0.47 –0.86), time to symptomatic progression (HR 0.77; 95% CI, 0.52–1.13), and time to worst pain progression (HR 0.94; 95% CI 0.67–1.31):
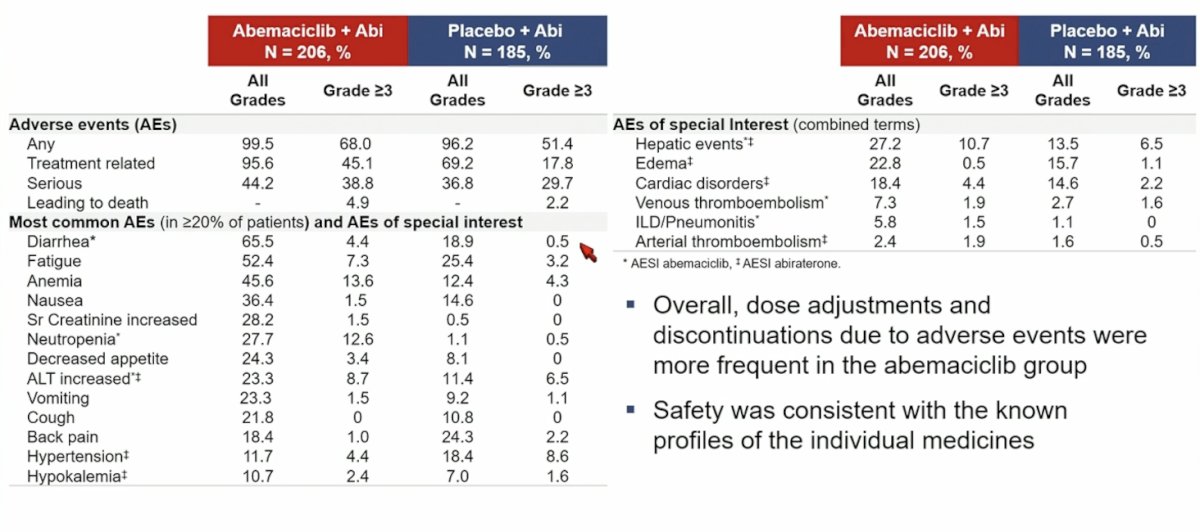
The most common grade ≥3 adverse events reported in the abemaciclib plus abiraterone group were anemia (13.6% vs 4.3% in the placebo plus abiraterone group), neutropenia (12.6% vs 0.5%) and ALT increased (8.7% vs 6.5%):
Discontinuations of all study treatments due to adverse events were 13.1% vs 4.3% in abemaciclib plus abiraterone vs placebo plus abiraterone groups, while discontinuations of abemaciclib or placebo alone due to adverse events were 5.8% vs 1.6%, respectively.
Dr. Smith concluded his presentation discussing results of the CYCLONE 2 trial with the following take home messages:
- The addition of abemaciclib to abiraterone did not significantly improve radiographic progression free survival in patients with mCRPC
- Safety of the combination was consistent with the known profiles of the individual treatments
Presented by: Matthew R. Smith, MD, PhD, Massachusetts General Hospital Cancer Center and Harvard Medical School, Boston, MA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, May 31st – June 4th, 2024