(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured an oral abstract session on prostate cancer, and a presentation by Dr. Christos Kyriakopoulos discussing results of the CHAARTED2 trial assessing cabazitaxel with abiraterone versus abiraterone alone for extensive disease following docetaxel. The E3805 (CHAARTED) trial previously showed a significant survival benefit from early treatment with chemohormonal therapy (ADT + docetaxel) in patients with high-volume metastatic hormone-sensitive prostate cancer (HSPC) [1]: 57.6 versus 44.0 months (p = 0.0003). Moreover, the STAMPEDE trial published shortly thereafter confirmed the findings in CHAARTED (HR 0.73, 95% CI 0.59-0.89) [2]. Additional longer follow-up of CHAARTED showed an overall survival benefit in high volume patients (HR 0.63, 95% CI 0.60-0.79) but not in low volume patients (HR 1.04, 95% CI 0.70-1.55) However, most patients will develop castration-resistant disease (CRPC) and will require additional systemic therapy. Dr. Kyriakopoulos and colleagues hypothesized that combining a second-generation AR inhibitor with a second-line chemotherapy could improve outcomes by eradicating clones with chemotherapy, thus prolonging the response to the AR inhibitor. At the time of trial design, there were two second generation AR inhibitors available (enzalutamide or abiraterone) and one second-line chemotherapy (cabazitaxel).
EA8153 (CHAARTED2) is a prospective randomized phase II open label trial. Patients with metastatic CRPC previously treated with ADT + docetaxel for HSPC were randomized (1:1) to abiraterone/prednisone plus cabazitaxel 25 mg/m2 for up to 6 cycles (n = 111) or abiraterone/prednisone alone (n = 112). Stratification factors included ECOG performance status of 0 versus 1-2, time from initiation of ADT to development of CRPC of <12 versus > 12 months, and presence versus absence of visceral metastases. The trial design for CHAARTED2 is as follows:
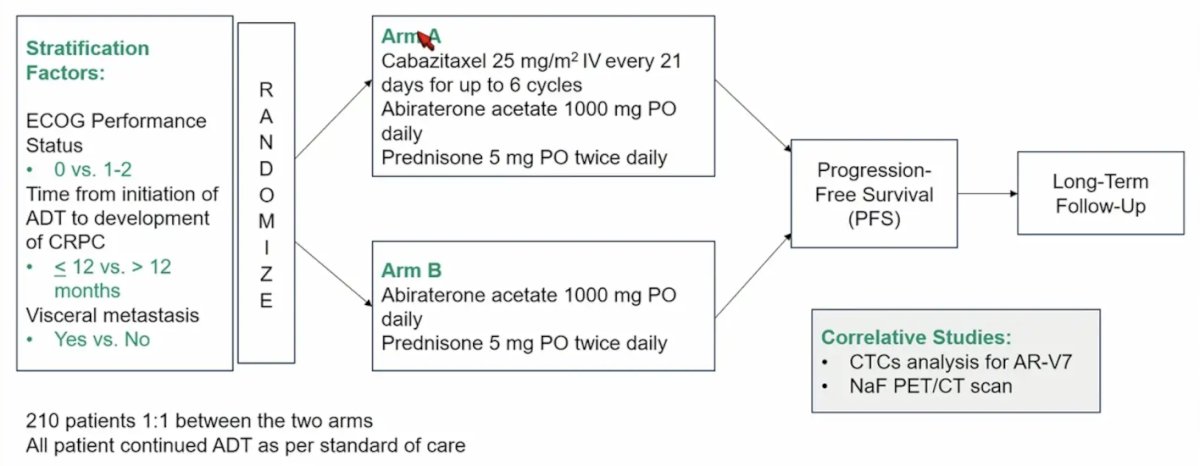
The primary trial endpoint is progression-free survival, defined as time from randomization to radiographic progression, symptomatic deterioration requiring discontinuation of treatment, or death. Key secondary endpoints include, PSA response, time to PSA progression, overall survival, safety and tolerability. Key inclusion criteria include mCRPC by PCWG3 criteria despite treatment with ADT, and prior treatment with at least 3 cycles of docetaxel for metastatic CSPC. Exclusion criteria are as follows:
- Any prior treatment for CRPC, including cabazitaxel, abiraterone or enzalutamide (radium-223 or sipuleucel-T were permitted)
- ECOG performance status > 2
- Grade >=2 peripheral neuropathy at baseline
From a statistical standpoint, a sample size of 210 patients is required based on a primary objective of 50% improvement in progression free survival with cabazitaxel, with a one sided type error of 0.1, power of 90%, hazard ratio of 0.67, and a planned full information at 166 progression free survival events. Efficacy interim analysis would be performed at 33% information (55 progression free survival events), and 67% information (111 progression free survival events). As of January 8, 2024, there were 223 patients randomized to the two arms, with 164 progression free survival events observed. A conditional power simulation was performed, and a higher hazard rate for Arm A was assumed. It was decided that the interpretation of the analysis would not change with the additional two events, thus this is the final analysis and the DSMC released the results.
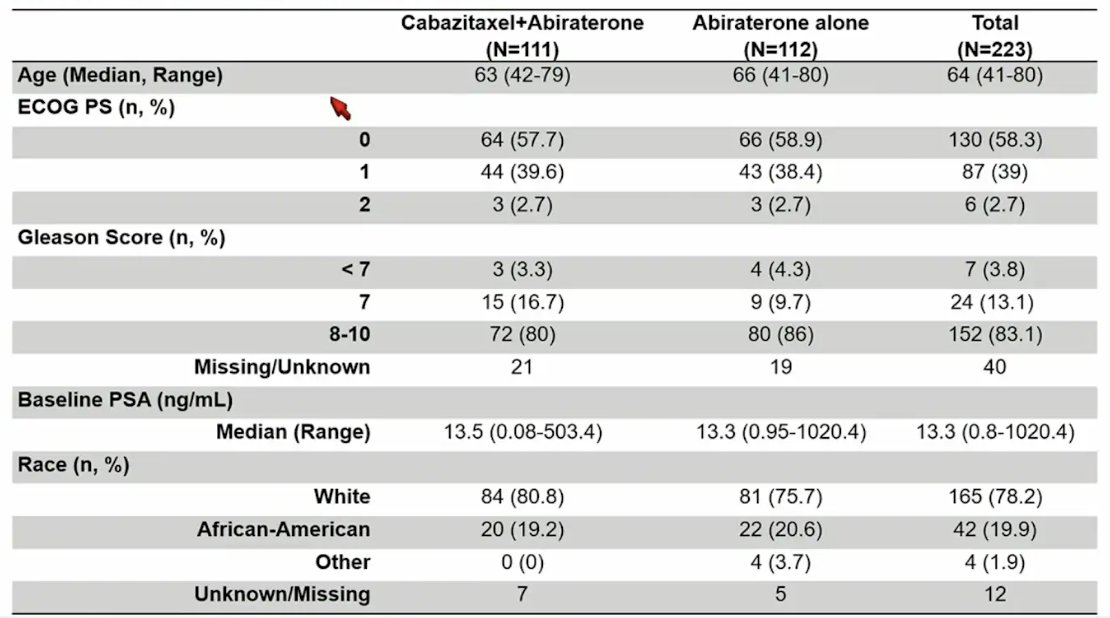
The baseline characteristics were well balanced between the two groups, with a median age of 64 years, 58.3% of patients ECOG 0, median baseline PSA of 13.3 ng/mL, 78.2% of patients were white, 19.9% of patients were African American, 76% of patients were high volume, and 100% of patients received docetaxel for mCSPC:

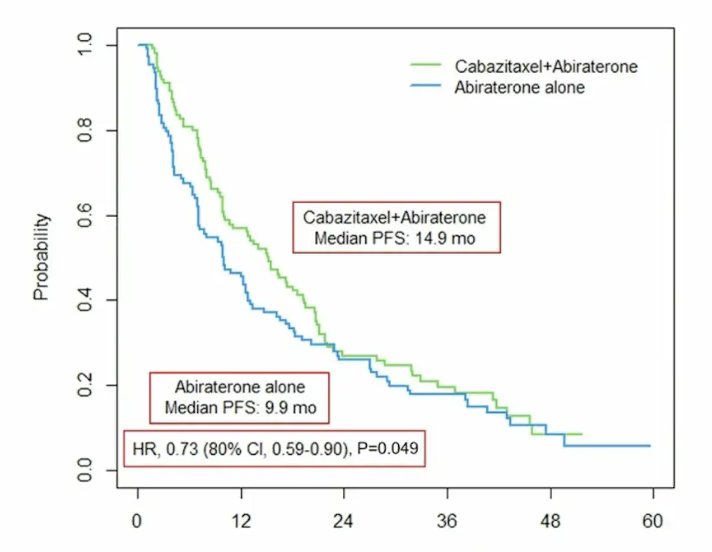
After a median follow-up of 47.3 (range: 0-61.2) months, median progression-free survival was longer for the cabazitaxel + abiraterone/prednisone arm versus abiraterone/prednisone alone arm (14.9 months [95% CI 9.9-18.6] versus 9.9 months [95% CI, 7.0-12.6], p = 0.049; HR 0.73, 80% CI 0.59-0.90):
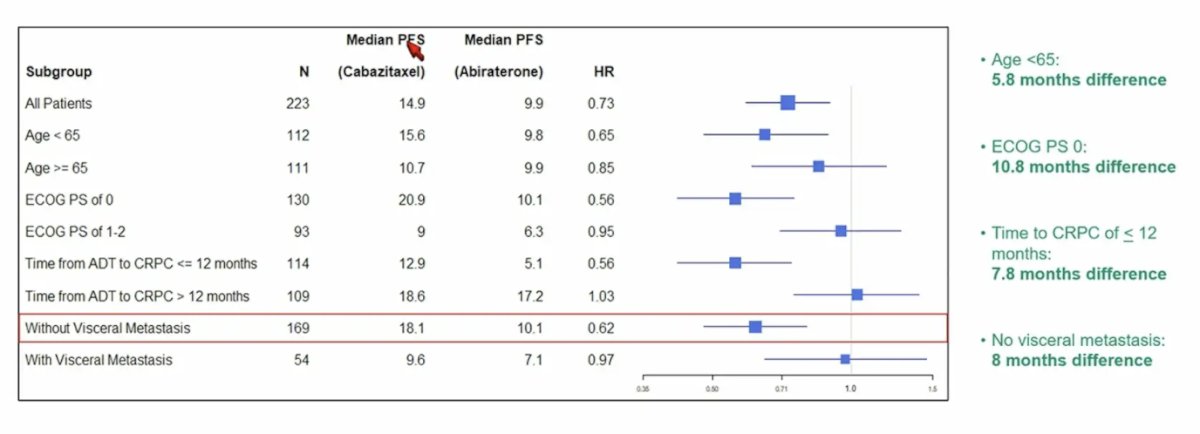
The advantage with the combination was more pronounced in patients < 65 years of age (15.6 versus 9.8 months, p = 0.08), ECOG performance status of 0 (20.9 versus 10.1 months, p = 0.01), time to CRPC of < 12 months (12.9 versus 5.1 months, p = 0.006), and absence of visceral metastases (18.1 versus 10.1 months, p = 0.01):
The PSA change from baseline to 12 weeks is as follows:
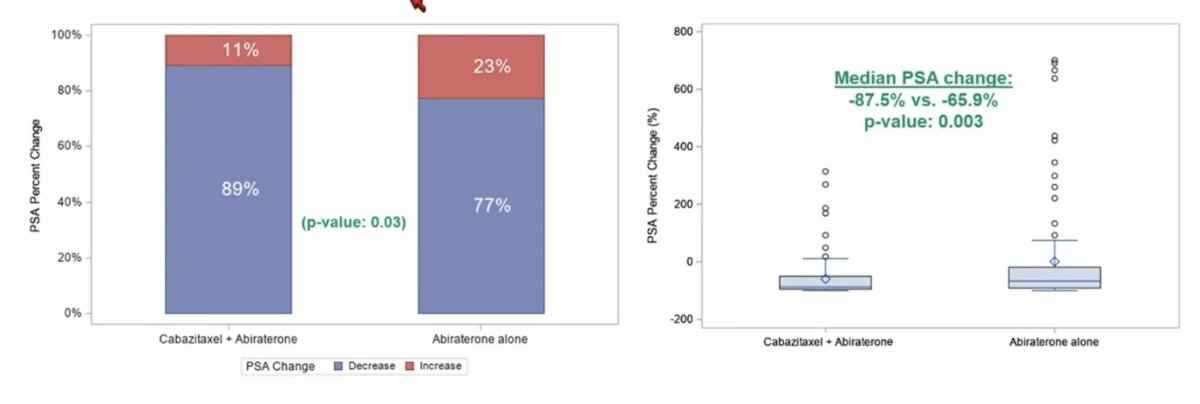
The median time to PSA progression was also longer in the combination versus the monotherapy arm (10 months [95% CI 8.5-13.5] versus 6.1 months [95% CI 4.4-8], p = 0.002).

No difference in overall survival was observed between the 2 arms in the interim analysis (25.0 versus 26.9 months; HR 0.93, 95 CI% 0.68-1.29; p = 0.67). More grade >3 side effects were noted in the combination arm, as expected from use of cabazitaxel. Treatment related toxicities of at least 5% in any of the two arms is as follows:

Treatment modifications include discontinuation, dose reduction or delay in therapy, highlighted as follows:
Dr. Kyriakopoulos concluded his presentation discussing results of the CHAARTED2 trial with the following take home messages:
- Cabazitaxel combined with abiraterone significantly prolonged progression free survival, improved PSA response, and delayed time to PSA progression compared to abiraterone alone in patients previously treated with docetaxel for metastatic CSPC, confirming the hypothesis that the combination may enhance the effect of single agent abiraterone
- There was no overall survival benefit, however the study was underpowered for this endpoint
- There were no significant safety concerns from the combination of cabazitaxel and abiraterone
- The changes in treatment landscape with the introduction of the doublet and triplet therapies in metastatic CSPC limit the applicability of these results in general practice
Presented by: Christos Kyriakopoulos, MD, University of Wisconsin Carbone Cancer Center, Madison, WI
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, May 31st – June 4th, 2024
Related content: CHAARTED 2 Study Shows PFS Benefit with Cabazitaxel in Metastatic Prostate Cancer in Phase 2 - Christos Kyriakopoulos
References:
- Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med. 2015;373(8):737-746.
- James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163-1177.
- Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: Long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol 2018 Apr 10;36(11):1080-1087.



