(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured a session on prostate cancer, and a presentation by Dr. Stephen Freedland discussing a post hoc analysis of EMBARK assessing the impact of treatment suspension on health-related quality of life.
EMBARK showed that enzalutamide + leuprolide and enzalutamide monotherapy delayed metastasis-free survival vs placebo + leuprolide while maintaining high global health-related quality of life in high-risk biochemically recurrent nonmetastatic hormone-sensitive prostate cancer.1 The FDA and the EMA recently added high-risk biochemical recurrence nonmetastatic hormone-sensitive prostate cancer as an indication for enzalutamide. Treatment was suspended at week 37 if the PSA was <0.2 ng/mL, and was reinstated if the PSA rose to ≥ 2.0 ng/mL with radical prostatectomy or ≥ 5.0 ng/mL without radical prostatectomy. This post hoc analysis presented at ASCO 2024 examined health-related quality of life after treatment suspension.
The study design for EMBARK is as follows:
Longitudinal change in health-related quality of life from the new baseline, week 37 (time of treatment suspension), to subsequent assessments (every 12 weeks) until week 205 were assessed via mixed model repeated measures using separate models for each instrument. For patients who reinitiated the treatment, assessments collected after reinitiating were excluded from the analysis. After week 109, the number of patients decreased considerably, and 95% confidence intervals became very wide, thus health-related quality of life data was presented at ASCO 2024 through week 109.
Treatment was suspended in 90.9% (321/353), 85.9% (304/354), and 67.8% (240/354) of patients treated with enzalutamide + leuprolide, enzalutamide monotherapy, and placebo + leuprolide, respectively. For BPI-SF item 3 (worst pain in the past 24 hours), there was no meaningful changes observed in any treatment arm after treatment suspension: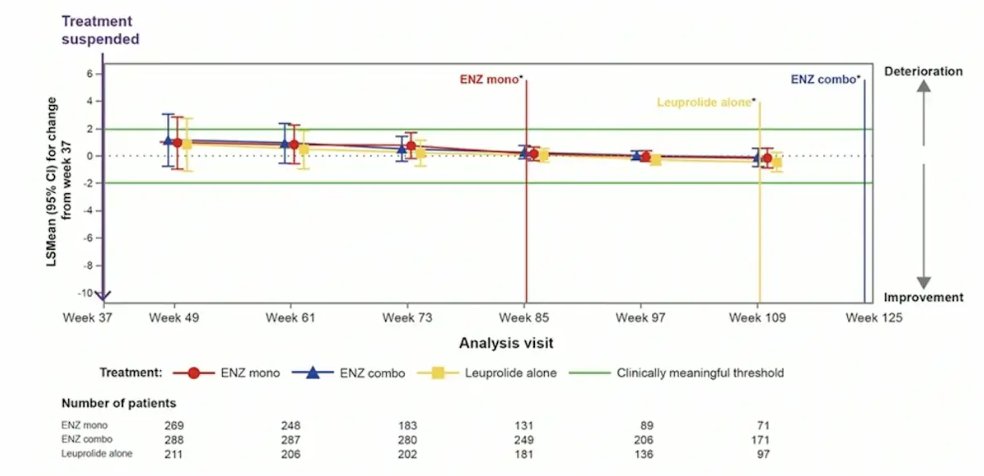
Similarly, for FACT-P total score, there was no meaningful changes observed in any treatment arm after treatment suspension: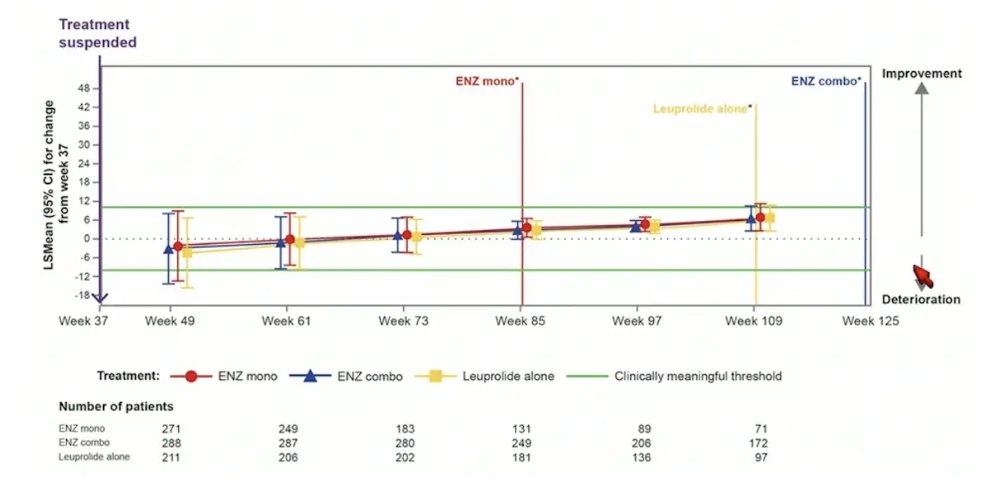
Moreover, for FACT-P physical well-being score, there was no meaningful changes observed in any treatment arm after treatment suspension:
Again, for EQ-5D visual analog scale score, there was no meaningful changes observed in any treatment arm after treatment suspension: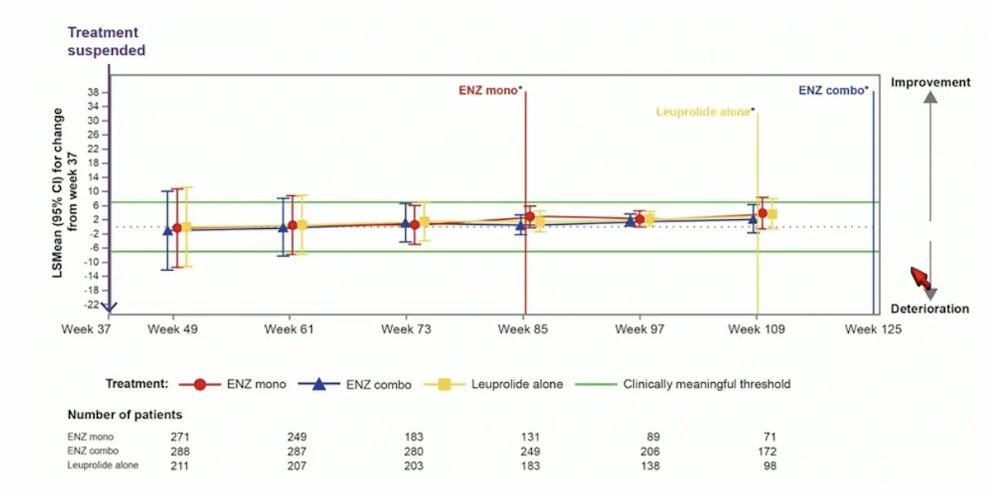
For QLQ-PR25 sexual activity score, there was no meaningful changes observed in any treatment arm after treatment suspension: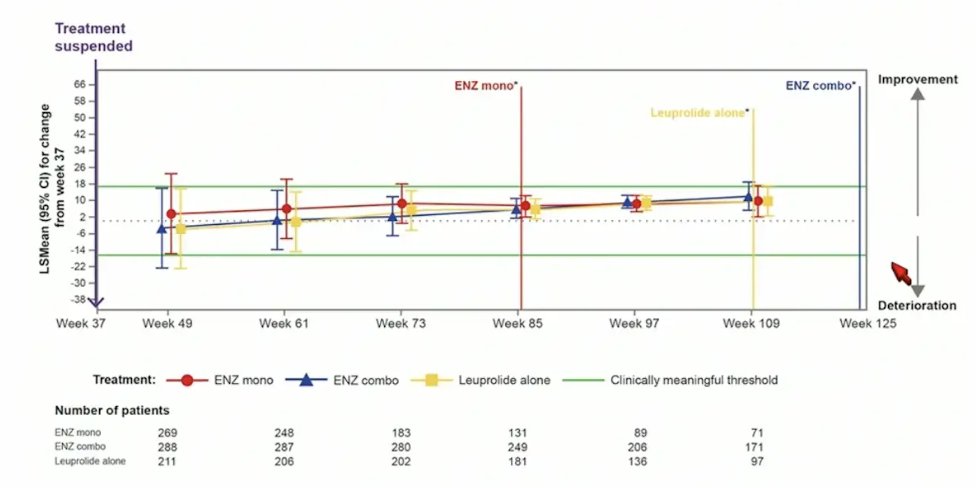
For QLQ-PR25 urinary symptoms score, there was no meaningful changes observed in any treatment arm after treatment suspension: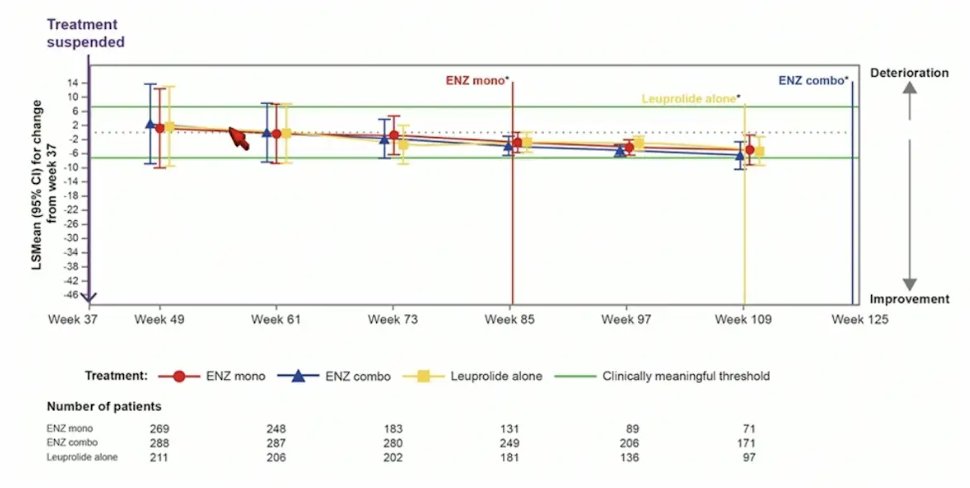
For QLQ-PR25 bowel symptoms score, there was no meaningful changes observed in any treatment arm after treatment suspension:
For QLQ-PR25 hormonal treatment-related symptoms, after treatment suspension, hormonal treatment-related symptoms quickly improved but eventually began to worsen after week 97: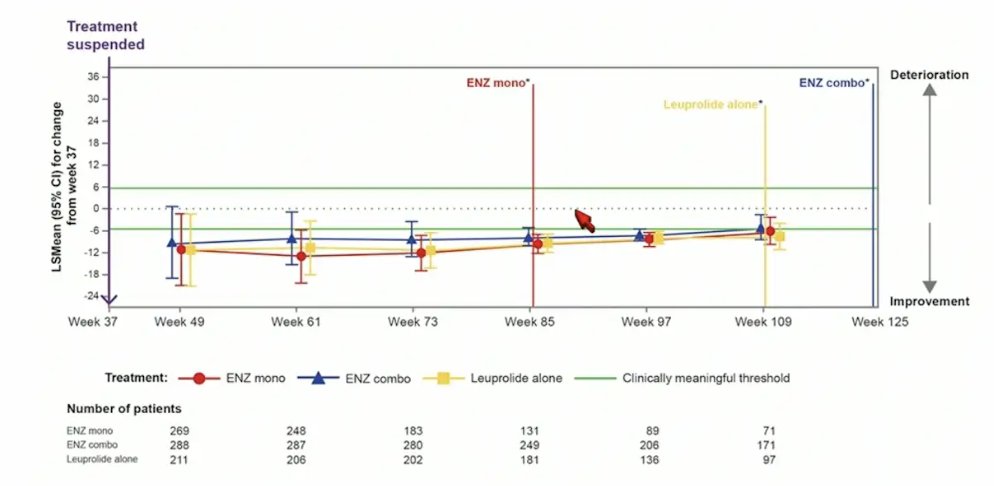
Limitations include patients not being randomized into the treatment suspension arms and sample size decreases over time with small sample sizes beyond week 109.
Dr. Freedland concluded his presentation discussing a post hoc analysis of EMBARK assessing the impact of treatment suspension on health-related quality of life with the following take-home messages:
- This post hoc analysis showed that after treatment suspension, hormonal treatment-related symptoms quickly improve in all arms, but worsen after week 97
- There were no clinically meaningful changes observed in other patients' reported outcome domains, reflecting minimal impact of treatment on global health-related quality of life
- These data, along with EMBARK clinical and patient-reported outcome data, show that enzalutamide with or without ADT, improves metastasis-free survival versus leuprolide alone, without affecting global health-related quality of life during treatment or after treatment suspension
Presented by: Stephen J. Freedland, MD, Director of the Center for Integrated Research in Cancer and Lifestyle, Associate Director for Training and Education at the Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinari, Los Angeles, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
References:


