(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured a session on prostate cancer, and a presentation by Dr. Ronald Chen discussing health-related quality of life results from PRESTO, a phase 3 randomized trial of intensification of androgen blockade in patients with high-risk biochemically relapsed castration sensitive prostate cancer.
Approximately 30% of men with localized prostate cancer treated with radical prostatectomy or radiotherapy will experience a biochemical recurrence. Intermittent ADT is a commonly used standard of care, which usually involves an initial course of ADT (6-12 months) followed by a period of being off treatment. Patients are restarted on ADT at pre-defined levels of PSA rise. Thus, there is an unmet need given that patients with a short PSA doubling time are at high risk for distant metastasis and prostate cancer-specific mortality. The PRESTO trial previously showed that in patients with biochemically relapsed prostate cancer following radical prostatectomy with a PSA doubling time of ≤ 9 months and without evidence of metastatic disease by conventional imaging, intensified androgen receptor blockade for 52 weeks (ADT plus apalutamide, or ADT plus apalutamide and abiraterone acetate plus prednisone) prolonged PSA progression-free survival compared to ADT alone.1 At the 2024 ASCO annual meeting, Dr. Chen and colleagues reported the health-related quality of life results from PRESTO.
The trial design for PRESTO is as follows: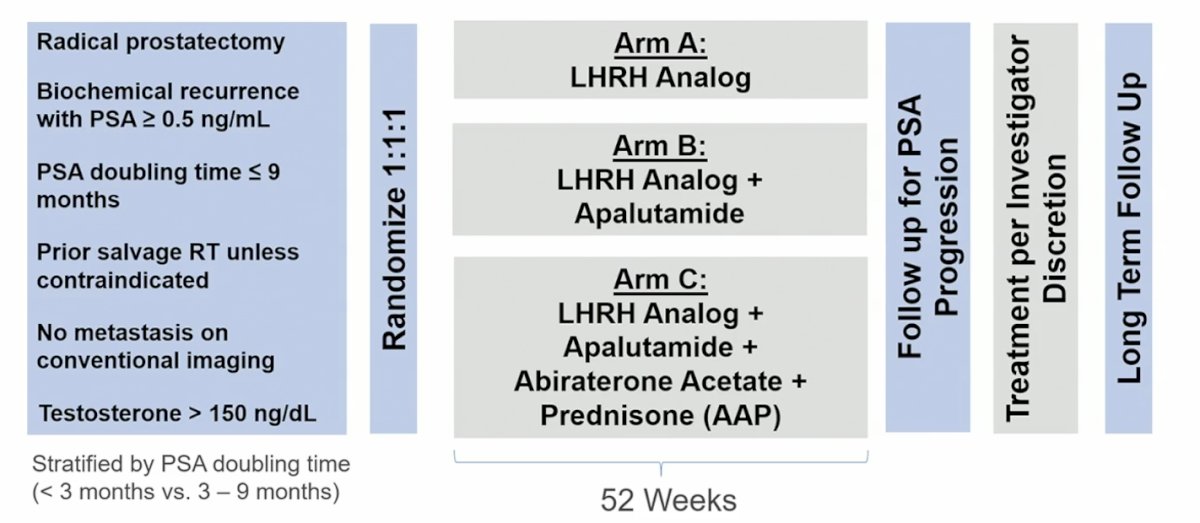
Although intensified androgen blockade improved PSA-PFS, patients, and physicians need to understand the impact on health-related quality of life to make an informed decision. Prior studies have assessed the health-related quality of life impact of apalutamide and abiraterone acetate plus prednisone in patients with advanced prostate cancer. Most prior studies assessed health-related quality of life using the FACT-P instrument, which is validated and commonly used for advanced prostate cancer. However, health-related quality of life impact of intensified androgen blockade in patients with earlier disease is understudied.
Health-related quality of life measures included the Hot Flash Related Daily Interference Scale, Expanded Prostate Cancer Index Composite (EPIC)-26 Sexual domain, PROMIS Fatigue Short Form, and EQ-5D-5L, which assessed overall health-related quality of life in the 504 randomized patients. Published minimally important difference thresholds for this health-related quality of life measures are Hot Flash Related Daily Interference Scale 1.66 points, EPIC Sexual 10-12, PROMIS Fatigue 3-5, and EQ-5D-5L 0.06. General linear mixed modeling was used to estimate between-arm mean differences in health-related quality of life according to an intent-to-treat approach.
The patient characteristics in the PRESTO trial are as follows: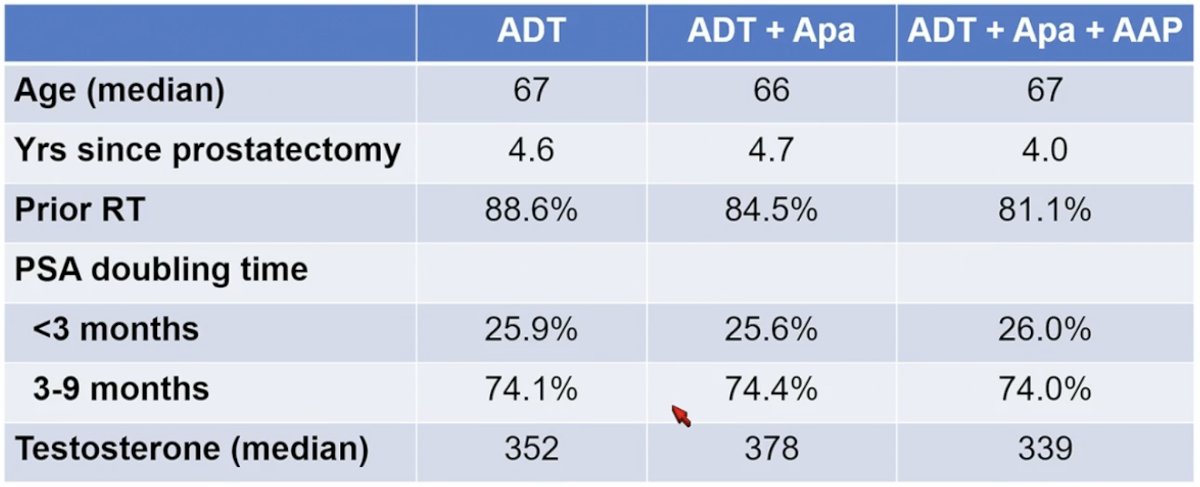
Treatment tolerability was generally quite good amongst all three arms, with 87-94% of patients completing treatment, and serious adverse events ranging from 8%-17%:
The EPIC sexual domain (0-100) showed no statistically significant differences between arms and no difference between arms above the minimally clinically important difference threshold:
Similarly, for the EPIC hormonal domain, no statistically significant differences between arms and no difference between arms above the minimally clinically important difference threshold: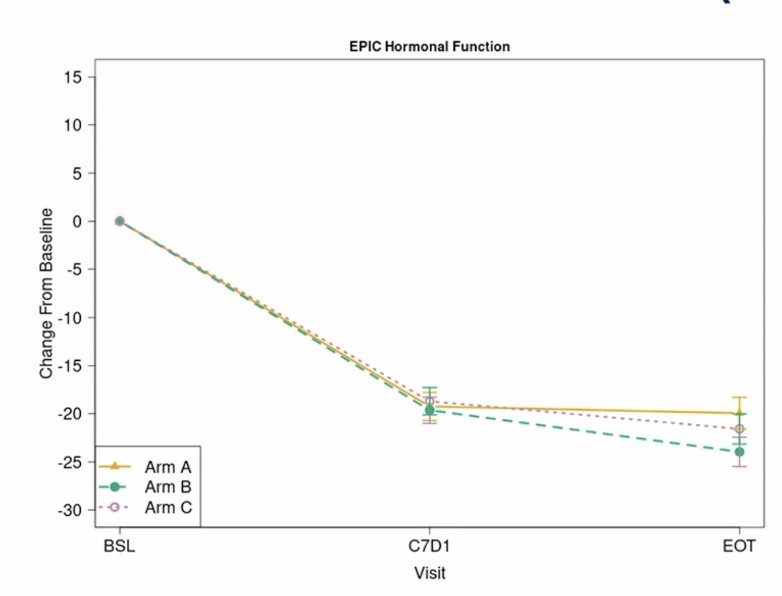
The individual EPIC hormonal domain individual items are as follows:
For the Hot Flash Related Daily Interference Scale, there was no statistically significant differences between arms, Arm C versus Arm A was above the minimally clinically important difference threshold: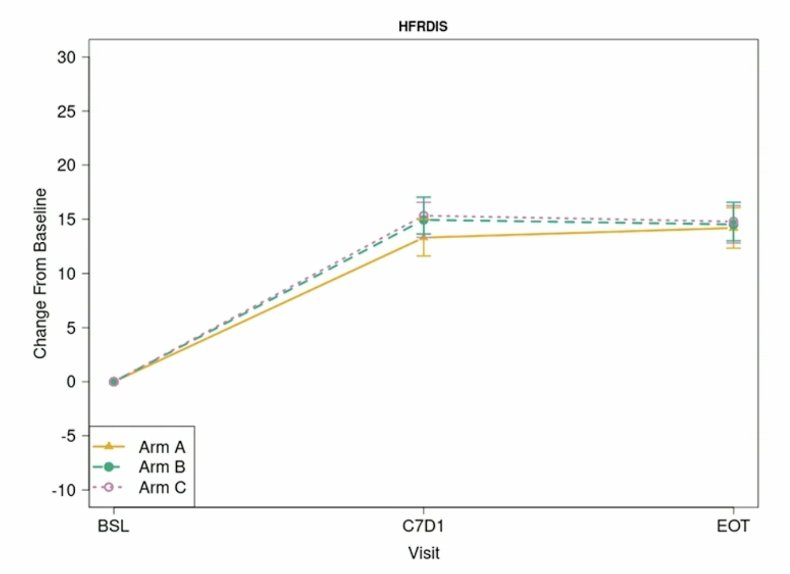
For PROMIS Fatigue, there was no differences between arms above the minimally clinically important difference threshold:
Finally, for EQ-5D-5L Index Score, there was no statistically significant differences between arms and no difference between arms above the minimally clinically important difference threshold:
Dr. Chen noted that limitations of this analysis include longer-term data being needed for providing insights on whether there are health-related quality of life changes after treatment cessation. This is needed to determine if improved PSA progression-free survival with intensified androgen blockade leads to measurable benefits in health-related quality of life due to decreased need for subsequent therapy. Key findings include (i) hormonal therapy meaningfully impacted hormonal symptoms, sexual function, hot flash interference, and fatigue, and (ii) the only finding that crossed the minimally clinically important difference threshold was hot flash interference for ADT + apalutamide + abiraterone versus ADT at cycle 7 day 1.
Dr. Chen concluded his presentation discussing health-related quality-of-life results from PRESTO with the following take-home messages:
- In men with biochemical recurrence after completing primary therapy (radical prostatectomy + adjuvant/salvage radiotherapy), intensified androgen blockade with apalutamide versus ADT alone improved PSA progression-free survival and:
- Did not increase serious adverse events
- Did not lengthen time to testosterone recovery
- Did not meaningfully increase common treatment-related symptoms, such as hormonal symptoms, sexual dysfunction, hot flash interference, or fatigue
- Additional intensification with triple androgen blockade did not further improve PSA progression-free survival but did increase serious adverse events, lengthened time to testosterone recovery, and increased hot flash interference
Presented by: Ronald C. Chen, MD, MPH, Radiologist, University of Kansas Medical Center, Kansas City, MO
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
References:


