(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured a session on prostate cancer, and a discussant presentation titled “Something Old, Something New, Something Borrowed” by Dr. Shahneen Sandhu discussing the following three abstracts: “Cabazitaxel with abiraterone versus abiraterone alone randomized trial for extensive disease following docetaxel: The CHAARTED2 trial of the ECOG-ACRIN Cancer Research Group (EA8153)” by Dr. Christos Kyriakopoulos, “CYCLONE 2: A phase 3 study of abemaciclib with abiraterone in patients with metastatic castration-resistant prostate cancer” by Dr. Matthew Smith, and “A randomized, double-blind, placebo-controlled trial of metformin in reducing progression among men on expectant management for low-risk prostate cancer: The MAST (Metformin Active Surveillance Trial) study” by Dr. Anthony Joshua.
Dr. Sandhu started by discussing Something Old and the CHAARTED2 trial data. Sustained androgen receptor signaling plays a major role in mCRPC, exemplified by genetic aberrations in androgen receptor driving resistance and underpinning the importance of ongoing suppression of the androgen receptor. Cabazitaxel1 and abiraterone2 improves progression free survival and overall survival in patients with mCRPC who have been previously treated with docetaxel. Moreover, cabazitaxel retains anticancer activity and improves progression free survival and overall survival compared with an androgen receptor pathway inhibitor in patients previously treated with docetaxel.3 The CHAARTED2 investigators posit that the combination of androgen receptor pathway inhibitor with cabazitaxel can maximize anti-tumor activity in patients with mCRPC previously treated with docetaxel. This hypothesis is based on the predicate of eradicating heterogeneous castrate resistant subclones given the different modes of action of these agents.
Dr. Sandhu notes that androgen receptor signal directly contributes to taxane resistance, thus cabazitaxel efficacy can be improved by simultaneous blocking the androgen receptor in enzalutamide resistant preclinical studies. Enzalutamide given with cabazitaxel induces enhanced apoptosis. Furthermore, gene set enrichment analysis shows repression of the androgen receptor signaling and several metabolic pathways, including fatty acid metabolism. In CHAARTED2, after a median follow-up of 47.3 (range: 0-61.2) months, median progression-free survival was longer for the cabazitaxel + abiraterone/prednisone arm versus abiraterone/prednisone alone arm (14.9 months [95% CI 9.9-18.6] versus 9.9 months [95% CI, 7.0-12.6], p = 0.049; HR 0.73, 80% CI 0.59-0.90). No difference in overall survival was observed between the two arms in the interim analysis (25.0 versus 26.9 months; HR 0.93, 95 CI% 0.68-1.29; p = 0.67). Additionally, the PSA change from baseline to 12 weeks is as follows:
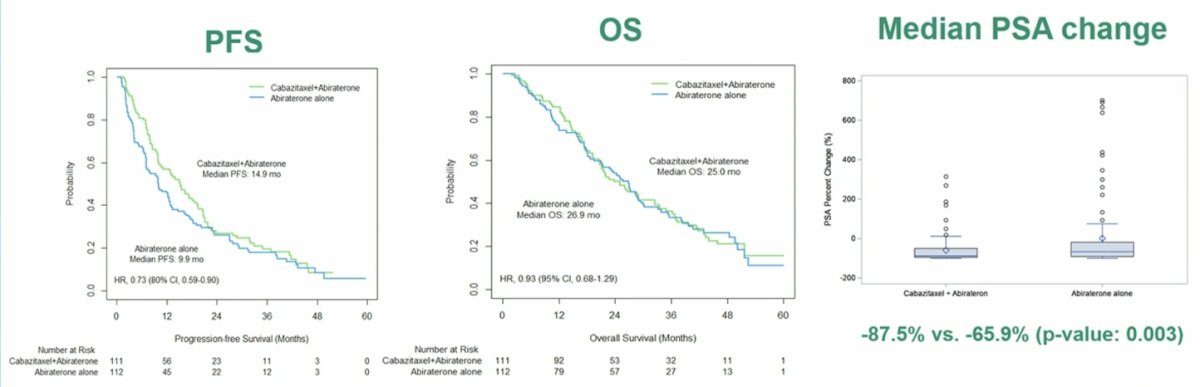
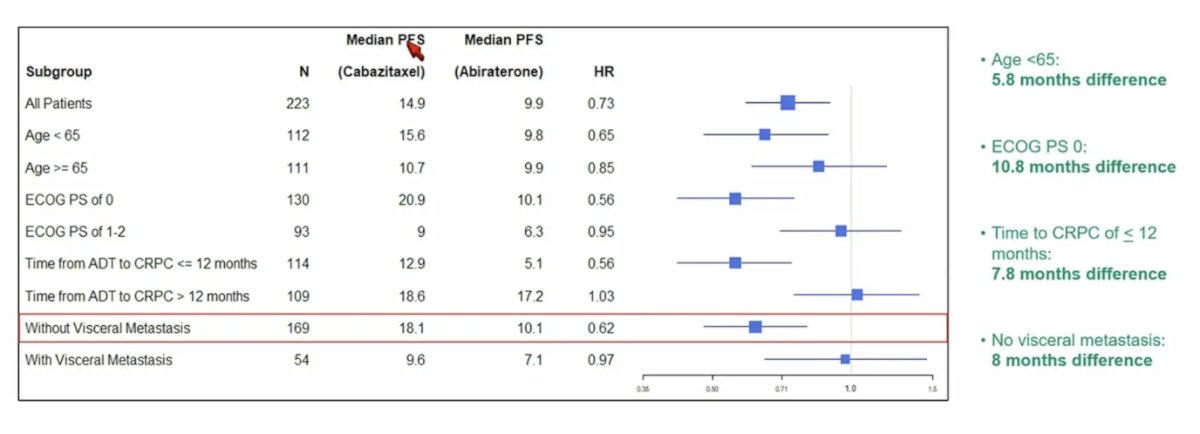
Looking at the PSA analysis by subgroups, the advantage with the combination was more pronounced in patients < 65 years of age (15.6 versus 9.8 months, p = 0.08), ECOG performance status of 0 (20.9 versus 10.1 months, p = 0.01), time to CRPC of < 12 months (12.9 versus 5.1 months, p = 0.006), and absence of visceral metastases (18.1 versus 10.1 months, p = 0.01):
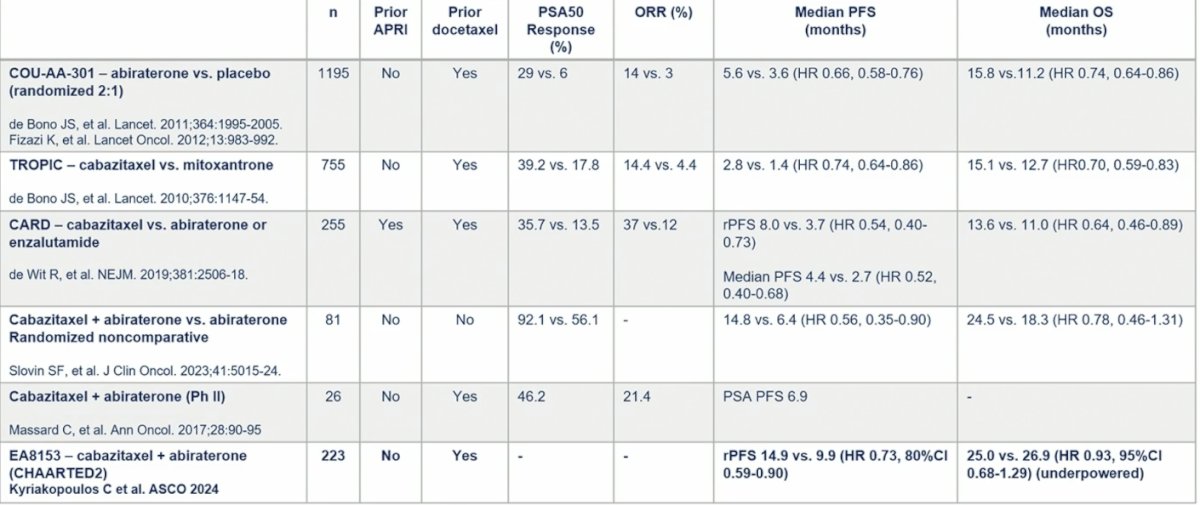
With regards to safety, there were no new signals, and grade 3+ adverse events were consistent with what was seen in the TROPIC study [1]. The following table provides insights from other trials with regard to the context of CHAARTED2:
Dr. Sandhu then provided several conclusions and takeaway points from the CHAARTED2 trial:
- There is preclinical, clinical, and biologic rationale that supports the role of cabazitaxel administered simultaneously with an androgen receptor pathway inhibitor in mCRPC
- The safety profile of cabazitaxel + abiraterone was consistent with each individual component and was generally manageable
- Unsurprisingly, cabazitaxel + abiraterone with different modes of action was more effective compared to single agent abiraterone, which is reflected in the improved progression free survival, PSA response, and delayed time to PSA progression
- It is clear that some patients benefit from treatment intensification of chemo-hormones with ADT, such as cabazitaxel + abiraterone, most notably those patients with a shorter response to ADT in mHSPC exhibiting the greatest benefit from treatment intensification in mCRPC
- However, the treatment landscape has shifted in recent years: androgen receptor pathway inhibitors are widely used for mHSPC with or without docetaxel, therefore these results have limited application in the clinic today
Dr. Sandhu then discussed Something New, specifically the CYCLONE 2 trial. The RB/E2F1 cell cycle pathway in tumor suppression is important, and CDK4 and CDK6 mediate the G1-S phase transition through phosphorylation of RB1. Successful inhibition of CDK4/6 signaling relies on an intact cyclin D-CDK4/6-RB1 axis, and cell type specific dependence on CDK4/6. CDK4/6 inhibition in combination with endocrine therapy has been practice changing for the treatment of advanced HR+HER2- breast cancer. CYCLONE 2 was a seamless phase 2/3 adaptive trial with a dose-finding safety lead-in (n = 46). Part 2 (n = 146) was expanded given a radiologic progression free survival advantage of HR < 0.668, and part 3 (n = 201) included enrollment expansion. Key eligibility included mCRPC, visceral metastases allowed (including liver metastases), ECOG performance status 0-1, and continuous ADT. Patients could not have prior treatment with CDK4/6 inhibitors, androgen receptor pathway inhibitors, or chemotherapy for mCRPC (prior docetaxel for mCSPC was permitted). Randomization to the abemaciclib or placebo plus abiraterone and predniso(lo)ne was stratified by prior docetaxel receipt for mHSPC, measurable disease, and radiographic progression at study entry. The trial design for CYCLONE 2 is as follows:
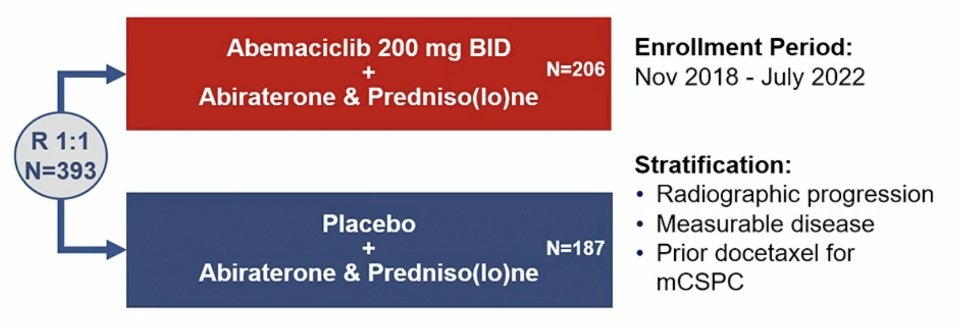
The primary endpoint was investigator-assessed radiographic progression-free survival per RECIST v1.1 and PCWG3 with the intention to treat population (Parts 1-3). Secondary endpoints included:
- Radiographic progression free survival by blinded independent central review
- Overall survival
- Objective response rate
- Duration of response
- Time to symptomatic progression
- Time to PSA progression
- Time to worst pain progression
- Safety
- Pharmacokinetics
This study found that there was limited efficacy from targeting CDK4/6 in mCRPC. In this trial, the primary endpoint of radiographic progression-free survival was not met (HR 0.83; 95% CI, 0.62–1.11; p = 0.212), with medians of 22.0 months for the abemaciclib plus abiraterone group versus 20.3 months for the placebo plus abiraterone group. Overall survival was a gated secondary endpoint and not inferentially tested (HR 0.93; 95% CI, 0.67–1.29; 38.9% maturity):
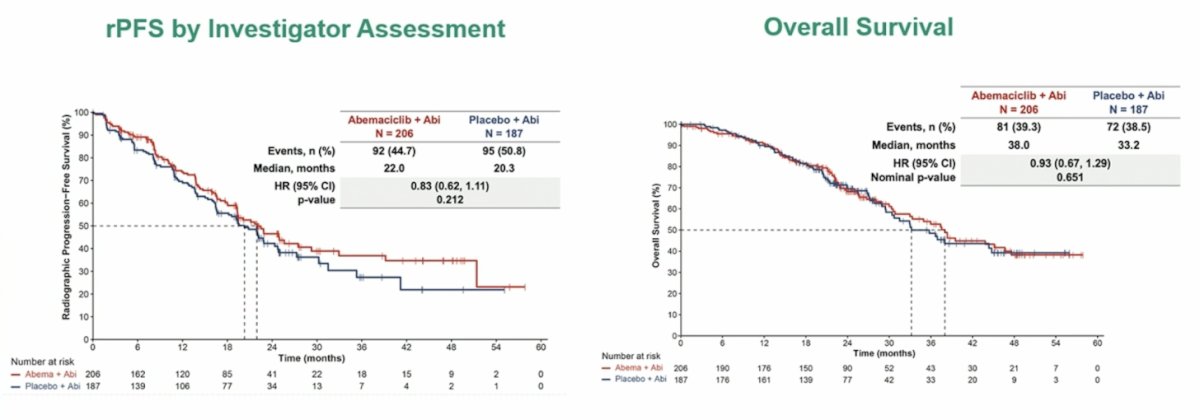
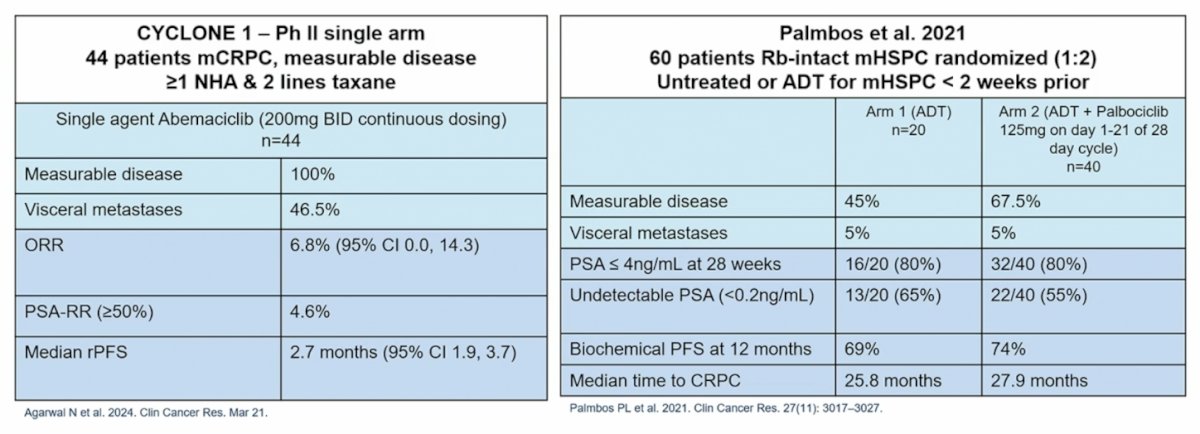
Additionally, there is other data to suggest that there is limited efficacy from targeting CDK4/6 in mCRPC and mHSPC, specifically from CYCLONE 14 and Palmbos et al.5:
Dr. Sandhu notes that there are several putative mechanisms of resistance to CDK4/6:
- RB loss: up to 10% of breast cancer patients with disease progression on CDK4/6 inhibitors acquire alterations or deletions of both copies of RB1, and loss of RB is common in mCRPC at ~30-60%
- Aurora kinase A: implicated in both intrinsic and acquired resistance to CDK4/6 inhibitors
- CDK6 amplifications with increased CDK6 expression promote resistance. CDK6 expression is also increased by the loss of PTEN, another common alteration in mCRPC
- c-MYC is upregulated in CDK4/6 inhibitor-resistant cell lines and acquired mutations in MYC were identified in 5-9% of patients with disease progression on abemaciclib, and MYC upregulation is common in mCRPC
- RB loss is the major mechanism of pathway disruption in advanced prostate cancer. Recent findings suggest that having RB loss induces distinct downstream E2F cistrome expansion with RB inactivation leading to a distinct transcriptional network that promotes disease aggressiveness
Dr. Sandhu’s key takeaway points from CYCLONE 2 are that (i) the addition of abemaciclib to abiraterone did not significantly improve radiographic progression free survival in patients with mCRPC, and (ii) the molecular underpinnings accounting for the lack of activity from targeting CDK4/6 in prostate cancer is poorly understood and needs to be the subject of future research.
Finally, Dr. Sandhu discussed Something Borrowed, specifically the MAST trial in low risk prostate cancer. There is ample retrospective evidence for assessing metformin in this trial and potentially repurposing metformin as an anticancer drug. MAST was a randomized double blind placebo controlled trial carried out in 12 centers across Canada. Eligible patients had biopsy-proven, low-risk, localized prostate cancer diagnosed within the past 6 months, with a Gleason score of < 6 observed in ≤1/3 of the total cores, less than 50% positivity in any one core, a PSA level of ≤10 ng/ml, and a clinical stage between T1c-T2a. Additionally, patients chose active surveillance as their primary treatment. Subjects that met eligibility criteria were randomly assigned (1:1) to receive metformin 850 mg BID or placebo for 3 years. All patients underwent repeat prostate biopsy at 18 and 36 months. The primary endpoint indicated was time to progression, defined as the earliest occurrence of primary prostate cancer therapy (ie. prostatectomy, radiation, hormonal therapy) or pathological progression (>1/3 of total cores involved, at least 50% of any one core involved, or Gleason pattern 4 or higher). The trial design for the MAST trial is as follows:
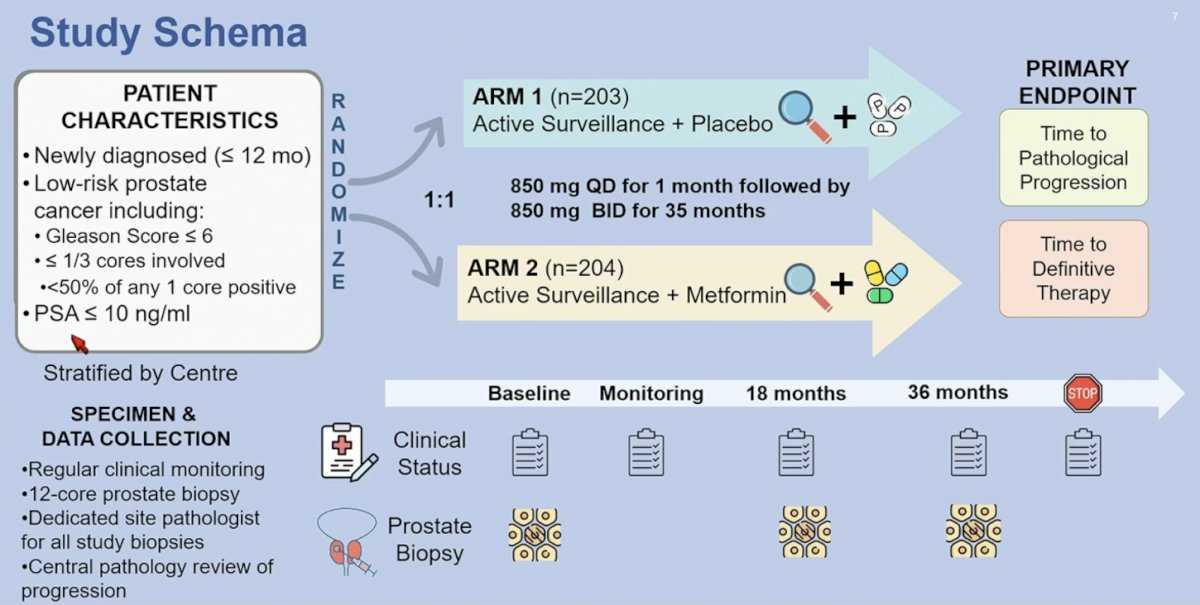
There was no statistically significant difference in progression-free survival observed between patients treated with metformin and those receiving placebo with regards to therapeutic + pathologic progression (HR 1.08, 95% CI 0.78-1.50), pathologic progression (HR 1.07, 95% CI 0.76-1.52), or therapeutic progression (HR 1.75, 95% CI 0.99-3.08):
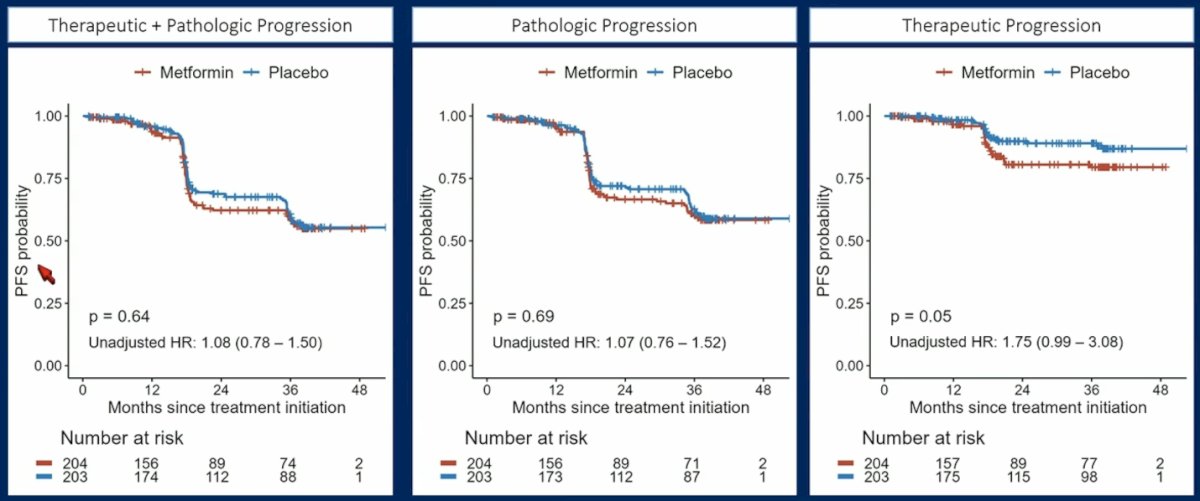
Taking a closer look at pathologic progression endpoints, there were no statistically significant differences, although Gleason >= 8 disease was more common in the metformin (12.9%) versus the placebo (4.5%) group (p = 0.082). Patients with a BMI >= 30 had a worse progression free survival probability (HR 2.39, 95% CI 1.20-4.75; interaction p = 0.01) with metformin:

Dr. Sandhu concluded her presentation with the following messages regarding the MAST trial:
- This study raises more questions than answers
- It is valuable to have a prospective study (despite the results) given much of the literature is focused on retrospective analyses, with metformin use usually being associated with type 2 diabetes mellitus
- Critically, the investigators have collected serial tumor biopsies which will be subjected to proteomic and metabolomic analyses and likely be quite informative
- There needs to be further investigation into metformin’s direct effects on tumor metabolism/microenvironment versus systemic alterations in metabolism that may have impacted on the pathologic differences in groups (ie. is this mTOR/insulin signaling alterations versus complex inhibition of the tumor?)
- We need to consider if there is a baseline tumor metabolic phenotype that would make metformin more or less likely to benefit or be harmed by this intervention
- The BMI impact on metformin effect is intriguing: is the increased BMI associated with some advantageous features within the tumor in this context that are altered/attenuated by metformin or is there a bigger impact from metformin on systemic metabolism in those with increased BMI?
Presented by: Shahneen Sandhu, Peter MacCallum Cancer Center and the University of Melbourne, Melbourne, Australia
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
References:
- de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010;376(9747):1147-1154.
- de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995-2005.
- de Wit R, de Bono J, Sternberg CN, et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. N Engl J Med 2019 Dec 26;381(26):2506-2518.
- Agarwal N, Castellano D, Alonso-Gordoa T, et al. A signal-finding study of Abemaciclib in heavily pretreated patients with metastatic castration-resistant prostate cancer: Results from CYCLONE 1. Clin Cancer Res. 2024 Mar 21 [Epub ahead of print].
- Palmbos PL, Daignault-Newton S, Tomlins SA, et al. A randomized phase II study of androgen deprivation therapy with or without palbociclib in RB-positive metastatic hormone sensitive prostate cancer. Clin Cancer Res. 2021 Jun 1;27(11):3017-3027.


