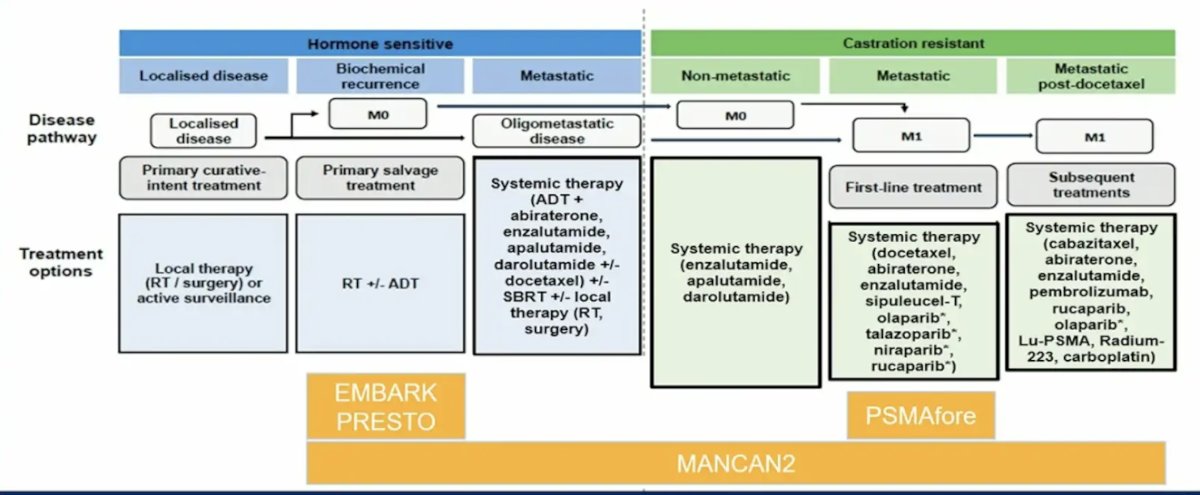
(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured a session on prostate cancer, and a discussant presentation by Dr. Channing Paller titled “Living Your Best Life on Treatment” discussing the following four abstracts: “Health-related quality of life and pain in a phase 3 study of 177Lu-PSMA-617 in taxane-naïve patients with metastatic castration-resistant prostate cancer (PSMAfore)” by Dr. Karim Fizazi, “MANCAN2: A multicenter randomized controlled trial of self-help cognitive behavioral therapy to manage hot flush and night sweat symptoms in patients with prostate cancer receiving ADT” by Dr. Simon Crabb, “EMBARK post hoc analysis of impact of treatment suspension on health-related quality of life” by Dr. Stephen Freedland, and “Health-related quality of life results from PRESTO (AFT-19), a phase 3 randomized trial of intensification of androgen blockade in patients with high-risk biochemically relapsed castration sensitive prostate cancer” by Dr. Ronald Chen. To put into context these four trials in the prostate cancer landscape, Dr. Paller started by highlighting the following figure:
There are several interventions for hot flashes and night sweats, including:
- Physical activities, behavioral therapies, and devices: acupuncture, exercise training, yoga, relaxation training, cognitive behavioral therapy, hypnosis, and thermoregulation devices
- Pharmacologic interventions: SSRIs, SNRIs, neuroleptic agents, anti-hypertensive agents, and oxybutynin (ASCO 2024 LBA 12004)
- Natural health products: flax, vitamin E, soy, ginseng, black cohosh
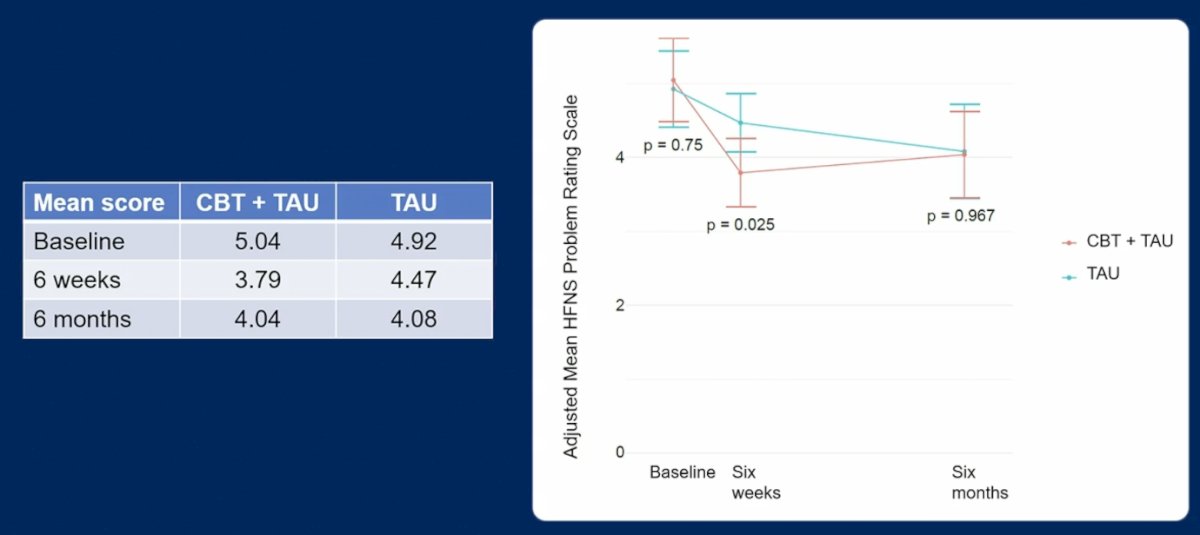
Cognitive behavioral therapy is psychological treatment that works by changing beliefs and improving an individual’s mood and sleep. The MANCAN trial was a single center randomized clinical trial of 68 prostate cancer patients, finding that self help cognitive behavioral therapy reduced the impact of ADT-associated hot flashes and night sweats at 6 weeks. In this context, the primary objective of MANCAN2 was to assess whether adding cognitive behavioral therapy to treatment as usual reduced 6 month hot flashes and night sweats problem rating scale versus baseline. MANCAN2 found that 6 month mean hot flush and night sweat problem rating scale score was not significantly different for the treatment as usual alone versus cognitive behavioral therapy + treatment as usual (mean 4.08 vs 4.04, 95% CI for difference: -0.89, 0.80; p = 0.97), although a difference was observed at 6 weeks (mean 4.47 vs 3.79, 95% CI: -1.26, -0.09; p = 0.03):
Dr. Paller’s conclusions of MANCAN2 are as follows:
- 4 weeks of self help cognitive behavioral therapy and relaxation strategies confirmed reduced hot flushes and night sweats at 6 weeks, but this was not maintained at 6 months
- Could continuing treatment or booster sessions extend the benefit beyond 6 weeks?
- Is such training practical and cost effective?
- This data is not currently practice changing
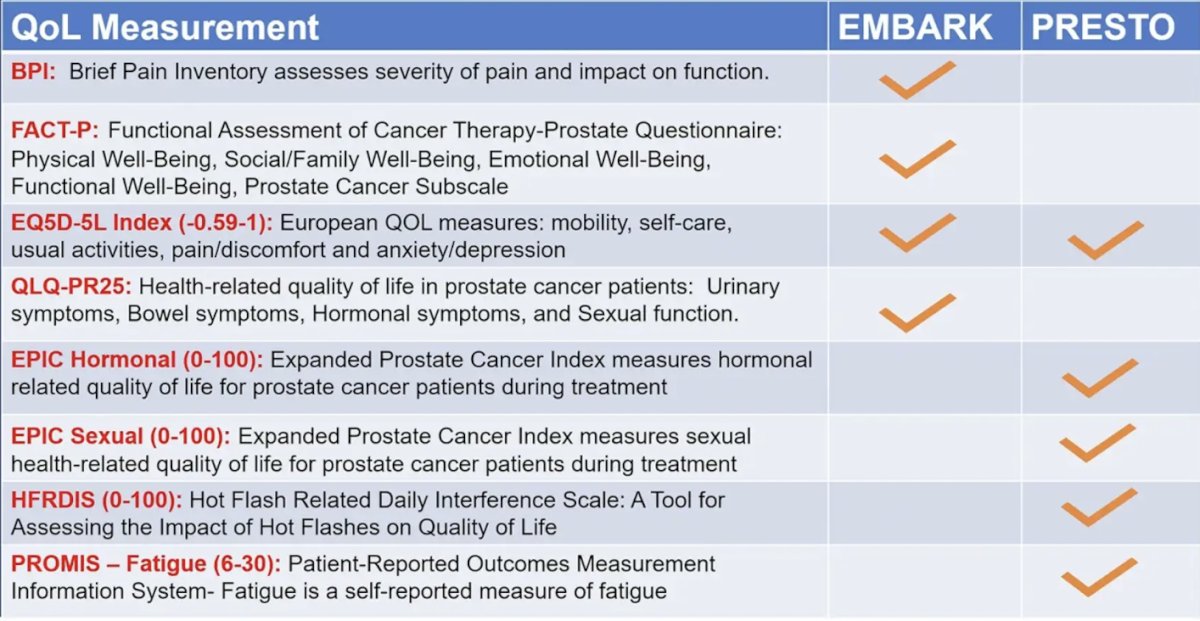
Dr. Paller then discussed the EMBARK trial,1 which showed that enzalutamide + leuprolide and enzalutamide monotherapy delayed metastasis-free survival versus placebo + leuprolide while maintaining high global health related quality of life in high-risk biochemically recurrent nonmetastatic hormone-sensitive prostate cancer. Given the plethora of quality of life metrics available, Dr. Paller highlighted the assessments used both in the EMBARK and PRESTO2 trials:
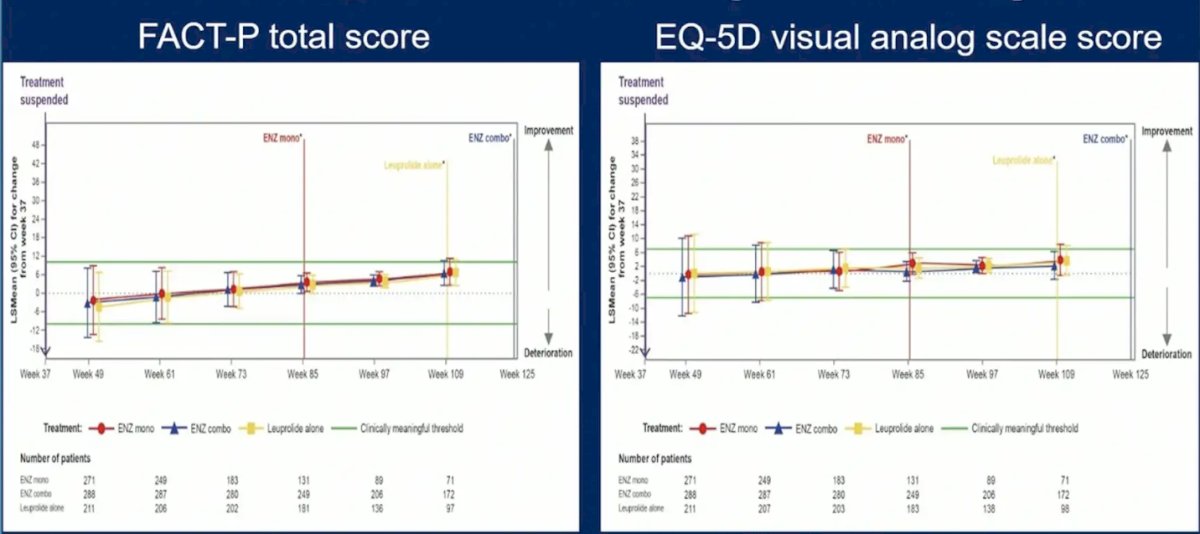
She also notes that in EMBARK treatment suspension resulted in no meaningful quality of life changes based on the FACT-P total score and the EQ-5D visual analog scale score:
However, Dr. Paller notes that it is important to look at the intention to treat data from the original reporting of the trial [3] to remember that several FACT-P metrics favored the leuprolide arm versus both the enzalutamide + leuprolide and enzalutamide monotherapy arms:

Moreover, there were meaningful changes in the experimental arms for hot flashes, fatigue, nipple pain, gynecomastia, ischemic heart disease, and cognitive impairment, particularly in the enzalutamide monotherapy arm:

Dr. Paller provided the following conclusions for the EMBARK analysis:
- Treatment intensification with enzalutamide is feasible without affecting global health related quality of life, although androgen receptor blockade alone has distinct side effects
- Regulatory approval of enzalutamide for non metastatic CSPC with high-risk features is based on the metastasis free survival and overall survival data, and patient selection is key. Some biochemical recurrent patients (longer PSA doubling time and lower PSA) can be safely monitored. Comorbidities and side effects must be taken into consideration to help the doctor and patient make more personalized treatment choices
- Could further quality of life analyses by subgroups in the 25% quartiles with the best or worst quality of life outcomes allow a more nuanced interpretation of the data?
Dr. Paller then discussed PRESTO, a phase 3 trial of androgen annihilation in patients with high risk biochemically relapsed prostate cancer. The trial design and endpoints of this trial are as follows:
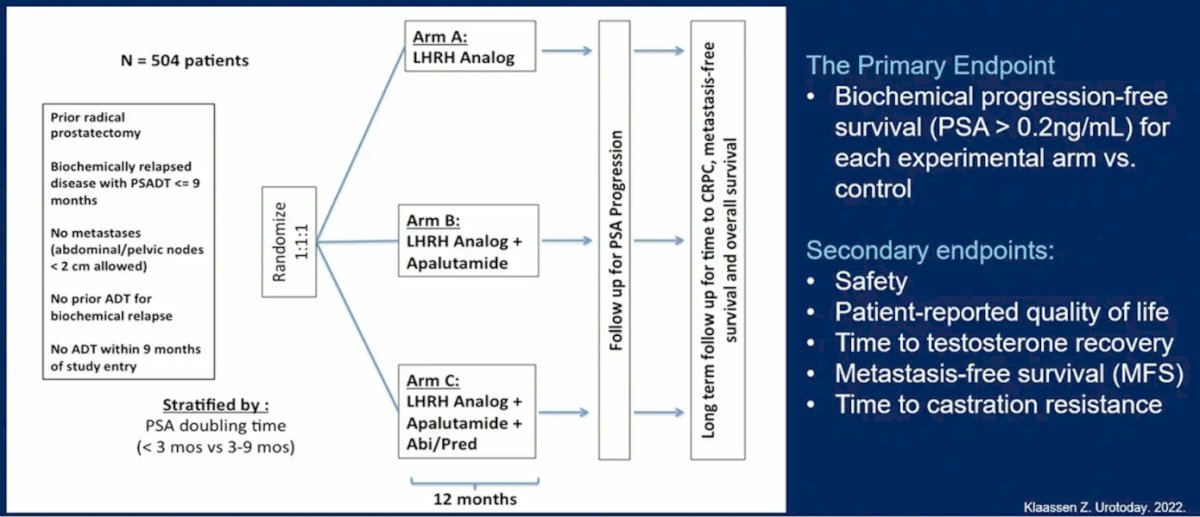
Importantly, the triplet combination of ADT + apalutamide + abiraterone had an above difference threshold at cycle 7 day 1 for the Hot Flash Related Daily Interference Scale:

Dr. Paller’s conclusions for PRESTO included:
- Intensification with apalutamide and ADT did not meaningfully increase common treatment related symptoms such as: hormonal symptoms, sexual dysfunction, hot flash interference, and fatigue
- Additional intensification with triplet therapy including apalutamide + abiraterone + ADT did not further improve PSA progression free survival, but did increase serious adverse events, lengthened time to testosterone recovery, and increased hot flash interference
- These findings are consistent with EMBARK and bolster the rationale for intensification with a different event profile
- PRESTO and EMBARK were not PET based and the biochemical recurrent patient population is likely to diminish
- What doctors and patients do with those PET images could potentially lead to overtreatment and worse quality of life
- As of now, this data is not practice changing
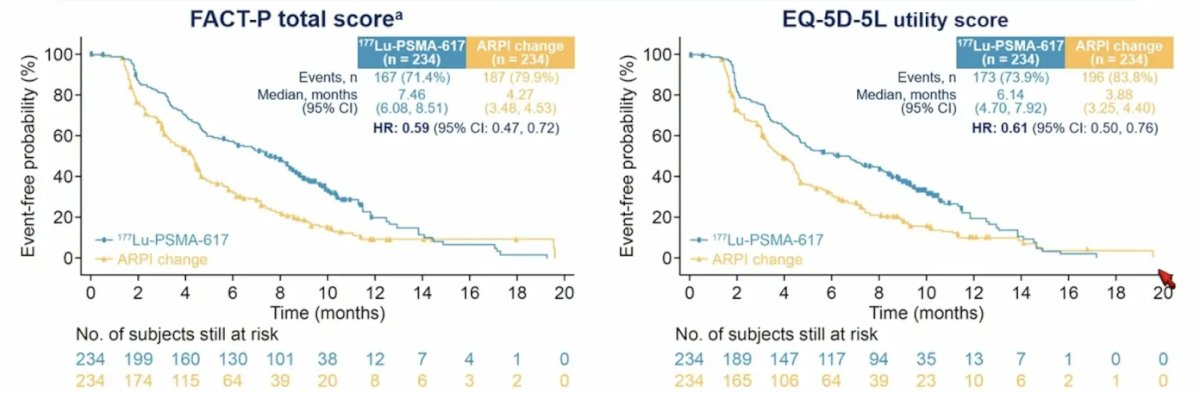
Finally, Dr. Paller discussed quality of life endpoints in the PSMAfore study. Eligible patients had mCRPC, were candidates for change of androgen receptor pathway inhibitor after progression on one prior androgen receptor pathway inhibitor, and had ≥1 PSMA-positive and no exclusionary PSMA-negative metastatic lesions by 68Ga-PSMA-11 PET/CT. Stratification factors included: (i) prior androgen receptor pathway inhibitor setting (castration resistant versus castration sensitive), and (ii) BPI-SF worst pain intensity score (0-3 vs > 3). PSMAfore randomized 468 patients (234 per arm). Median duration of exposure was 8.4 months for 177Lu-PSMA-617 and 6.5 months for androgen receptor pathway inhibitor change. 177Lu-PSMA-617 delayed time to worsening in FACT-P (HR 0.59, 95% CI 0.47-0.72) and EQ-5D-5L (HR 0.61, 95% CI 0.50-0.76):
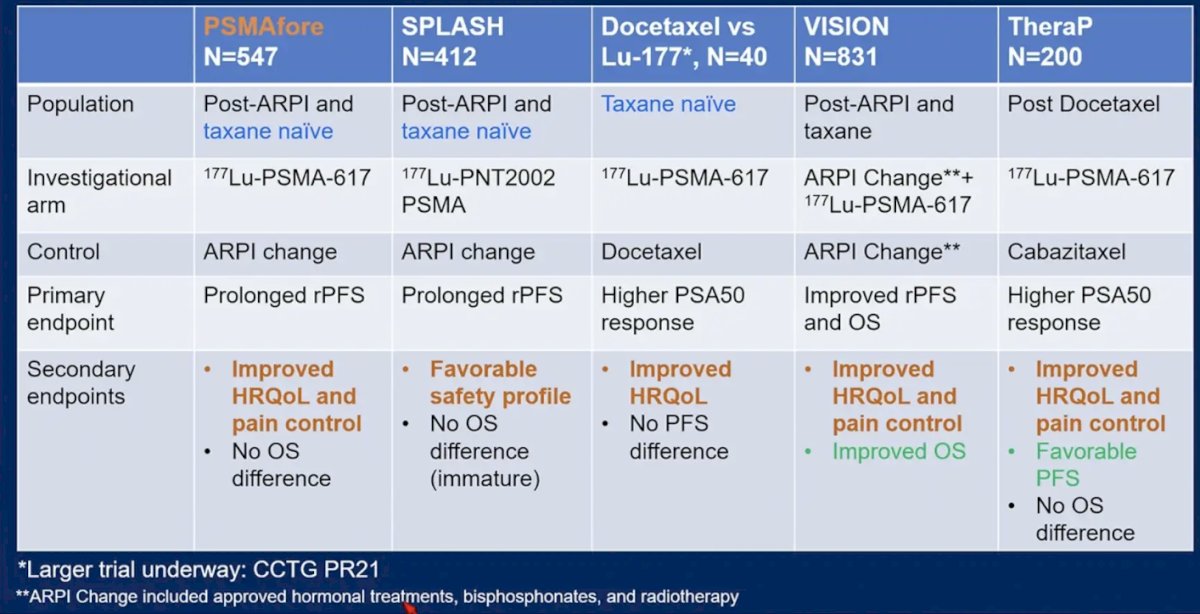
PSMAfore is the latest radioligand therapy trial to improve health related quality of life and pain control, as highlighted by the following table:
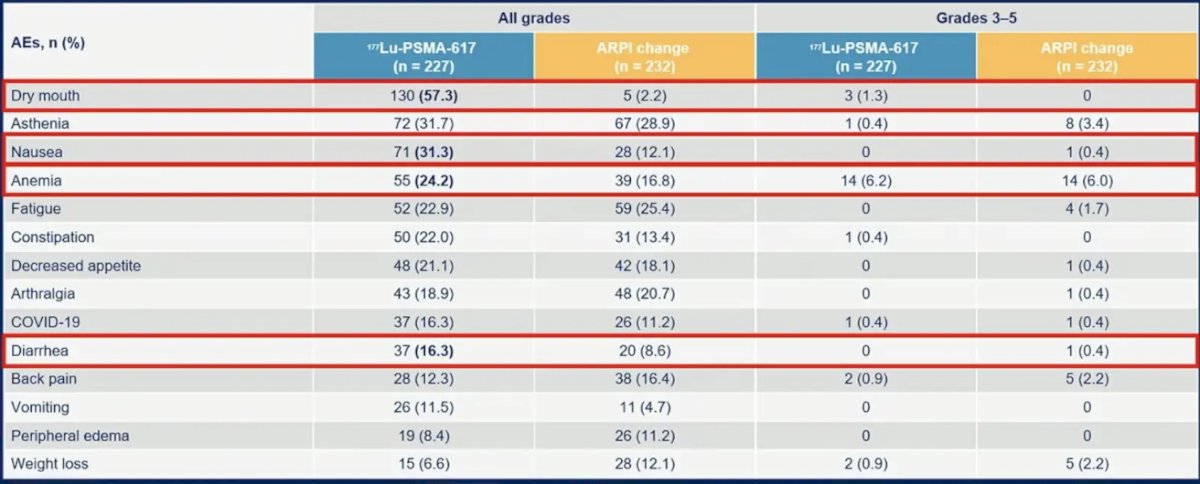
However, it is important to remember that 177Lu-PSMA-617 still leads to a substantial burden of dry mouth, nausea, anemia, and diarrhea:
Furthermore, Dr. Paller highlighted that we still need to understand the long term nephrotoxicity with 177Lu-PSMA-617. Recent work from Steinhelfer et al.4 in Germany suggests that the median percent eGFR decrease from baseline among patients treated with 177Lu-PSMA-617 is 13.6% at 12 months, 19.6% at 24 months, and 38.9% at 36 months. Risk factors associated with higher percent eGFR decreases included hypertension, diabetes mellitus, age > 65 years, and prior platinum chemotherapy. The number of pretreatment lines or cycles of 177Lu-PSMA-617 was not associated with nephrotoxicity. Additionally, it is important to note that nephrotoxicity can have a clinical impact when considering platinum based chemotherapy or PARP inhibitors. Dr. Paller’s conclusions for the quality of life analysis of PSMAfore are as follows:
- The quality of life data support 177Lu-PSMA-617 as a treatment option for patients with mCRPC who have undergone androgen receptor pathway inhibitor treatment and are not immediate candidates for chemotherapy
- More data is needed to provide guidance on sequencing of 177Lu-PSMA-617 and docetaxel
- We are awaiting the overall survival data of a small phase II trial
- We are also awaiting the results of CCTG PR21, a randomized phase II study of 177Lu-PSMA-617 versus docetaxel in patients with mCRPC and PSMA positive disease (NCT04663997)
- If approved pre-chemotherapy, this would also be practice changing
Presented by: Channing Judith Paller, MD, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
References:
- Freedland SJ, de Almeida Luz M, De Giorgi U, et al. Improved Outcomes with Enzalutamide in Biochemically Recurrent Prostate Cancer. N Engl J Med 2023 Oct 19;389(16):1453-1465.
- Aggarwal R, Heller G, Hillman DW, et al. PRESTO: A Phase III, Open-Label Study of Intensification of Androgen Blockade in Patients with High-Risk Biochemically Relapsed Castration-Sensitive Prostate Cancer (AFT-19). J Clin Oncol. 2024 Apr 1;42(10):1114-1123.
- Freedland SJ, Gleave M, De Giorgi U, et al. Enzalutamide and Quality of Life in Biochemically Recurrent Prostate Cancer. NEJM Evid. 2023 Dec;2(12):EVIDoa2300251.
- Steinhelfer L, Lunger L, Cala L, et al. Long-term nephrotoxicity of 177Lu-PSMA radioligand therapy. J Nucl Med. 2024 Jan 2;65(1):79-84.


