(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago, IL was host to a prostate, testicular, and penile cancers trials in progress poster session. Dr. Abhishek Solanki presented VA STARPORT, a Veterans Affairs (VA) seamless phase II/III randomized trial of standard systemic therapy with or without PET-directed local therapy for oligometastatic prostate cancer.
Dr. Solanki noted that the ‘oligometastatic’ concept in prostate cancer has shifted from a hypothetical/theoretic one to now having a ‘clear signal’. Multiple phase II trials have demonstrated that metastasis-directed therapy (MDT) using radiation or surgery is associated with oncologic benefits in patients with oligometastatic prostate cancer. However, these benefits have been primarily demonstrated in oligorecurrent prostate cancer patients (≤5 metastases) who had not received concurrent systemic therapy and had been conventional imaging-selected.
The incorporation of PSMA PET imaging potentially allows for earlier detection of oligometastases. Multiple phase III trials have demonstrated that earlier, aggressive, “enhanced” systemic therapy improves survival in metastatic prostate cancer, creating today’s standard systemic therapy. In this trial, Dr. Solanki and colleagues hypothesized that adding PET-directed local therapy to standard systemic therapy may improve disease control outcomes in veterans with oligometastatic prostate cancer.
This trial is randomizing 464 patients with either de novo or recurrent oligometastatic prostate cancer (≤10 lesions on PET/CT) planned for standard systemic therapy 1:1 to either:
- Standard systemic therapy alone
- Delivered with an intent for indefinite therapy using any NCCN guideline-concordant regimen in both arms.
- De novo patients: prostate-directed radiation per STAMPEDE
- Standard systemic therapy + PET-directed local therapy
- De novo patients: prostate-directed definitive radiation or radical prostatectomy + MDT
- Recurrent patients: treatment of any prostate/prostate bed local recurrence
- Metastasis-directed radiation can consist of stereotactic body radiotherapy or elective nodal radiotherapy with simultaneous integrated boost per clinician discretion from dose/fractionation options defined in the protocol.
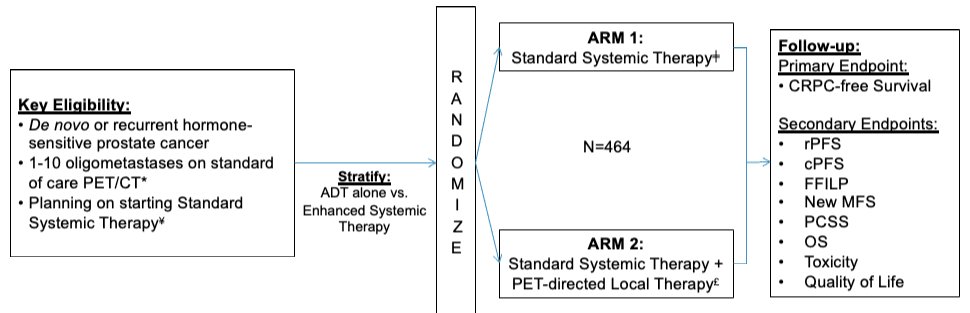
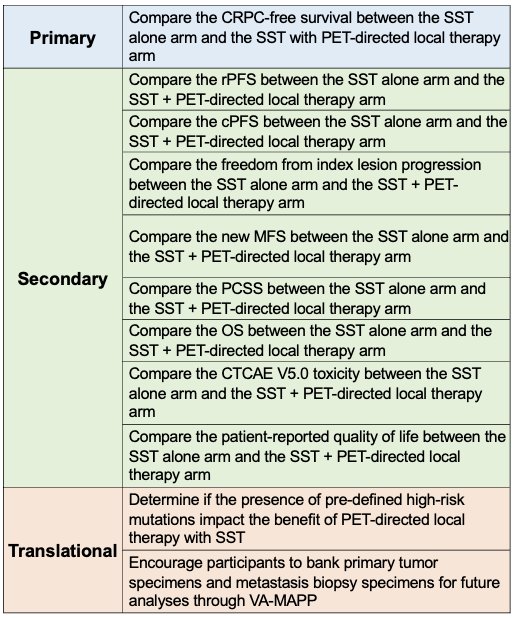
The primary study endpoint is castrate-resistant-free survival. The secondary and translational study objectives are summarized below:
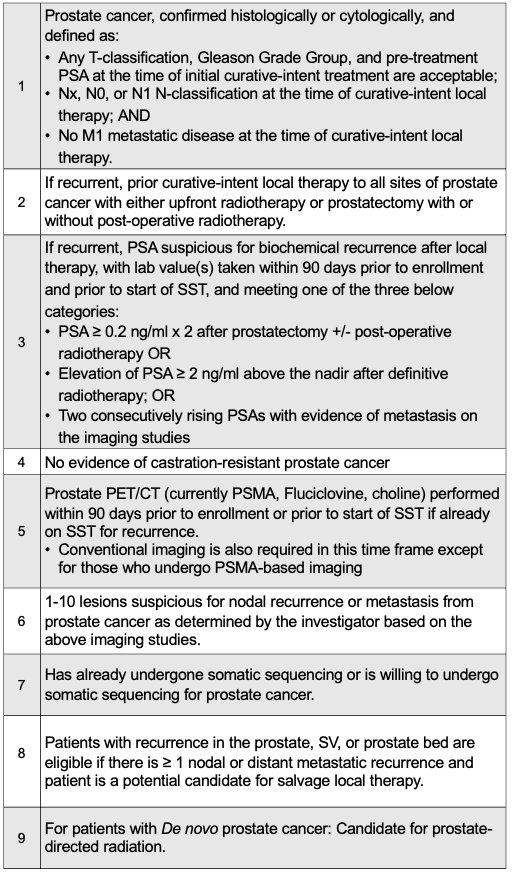
The key study inclusion criteria are summarized below:
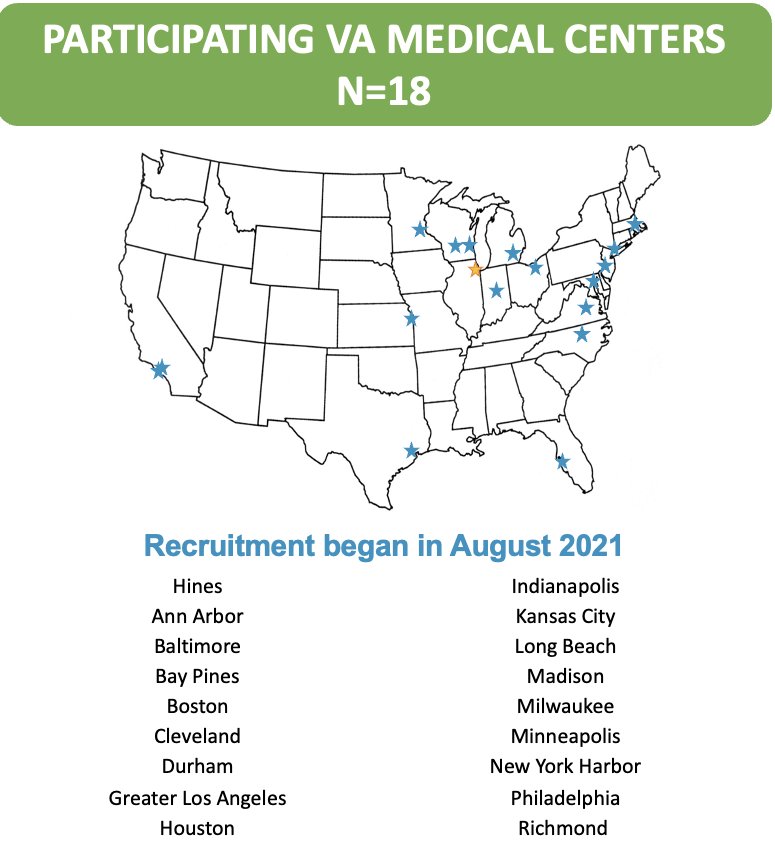
This study opened for recruitment in August 2021, and there are currently 18 VA participating sites.
Presented by: Abhishek A. Solanki, MD, MS, Associate Professor, Department of Radiation Oncology, Loyola University, Chicago, IL
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.


