(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago, IL between May 31st and June 4th, 2024 was host to a prostate, testicular, and penile cancers trials in progress poster session.
Dr. Oliver Sartor presented ProstACT GLOBAL, a phase 3 study of best standard of care with and without 177Lu-DOTA-rosopatamab (TLX591) for patients with PSMA expressing metastatic castration-resistant prostate cancer (mCRPC) progressing despite prior treatment with a novel androgen axis drug inhibitor.
TLX591 is a first-in-class radio-antibody drug conjugate (rADC) for prostate-specific membrane antigen (PSMA)-expressing mCRPC.
TLX591 utilizes a PSMA-targeted monoclonal antibody (mAb) approach. mAbs are distinguished by their internalization, long retention, and functional selectivity for tumor-expressed PSMA. Two treatments are administered 14 days apart. This minimizes the occurrence of off-target side effects while delivering a meaningful therapeutic index. TLX591 has been evaluated in more than 240 patients across eight phase 1–2 studies and has demonstrated proven anti-tumor effects with overall survival benefits. In a phase 1/2 study of 49 men with mCRPC refractory to or refusing standard treatment options, TLX591 demonstrated any PSA decrease in 88% of patients with a median survival of 42.3 months.1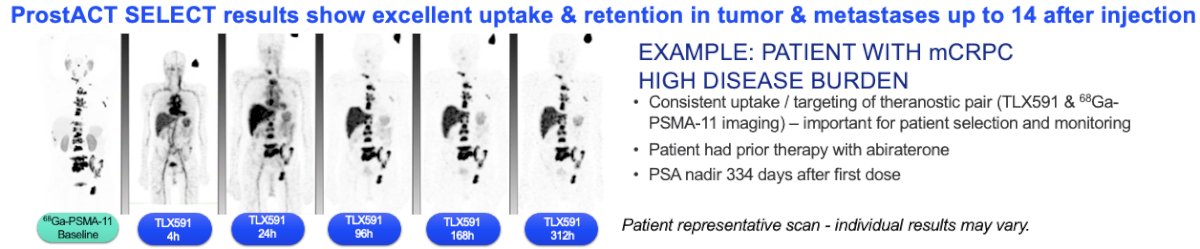
ProstACT GLOBAL is a prospective, open-label phase 3 trial of 430 patients with PSMA-expressing mCRPC who have experienced disease progression on prior androgen receptor pathway inhibitor (abiraterone acetate or enzalutamide only) received in either the metastatic castrate sensitive (de novo or recurrent) or first-line mCRPC treatment setting. Patients may have received docetaxel in the castrate-sensitive setting provided that the last dose of therapy was ≥6 months prior to screening.
The study design is summarized below. Part 1 will enroll 30 patients in the safety and dosimetry lead-in phase. The primary endpoints are biodistribution/pharmacokinetics, dosimetry, safety, and tolerability. In part 2 (randomized treatment expansion), 400 patients will be randomized 2:1 to either TLX591 + standard of care versus standard of care alone. The primary endpoint is radiographic progression-free survival, with key secondary endpoints of overall survival and safety.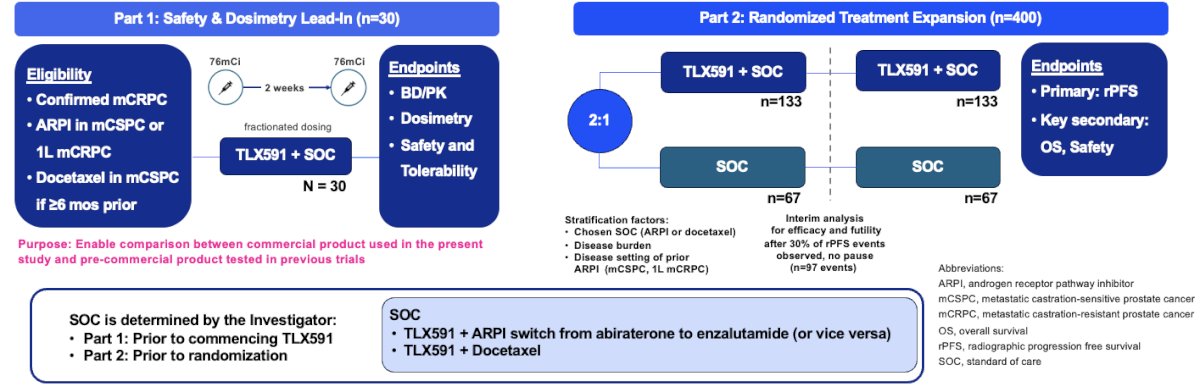
Standard of care is determined by the investigator prior to commencing TLX591 in Part 1 and prior to randomization in Part 2.
The key inclusion criteria are as follows:
- Adult patients with histologically/pathologically confirmed mCRPC (defined as ≥1 metastatic lesions present on baseline CT, MRI, or bone scintigraphy)
- A disease that is PSMA positive, as demonstrated by a 68Ga-PSMA-11 PET/CT scan
- ECOG Performance Status 0 – 2 with an estimated life expectancy ≥6 months
- Previously treated with first androgen receptor pathway inhibitor (abiraterone or enzalutamide) in the mCSPC (de novo or recurrent) or 1st line mCRPC setting for a minimum of 12 weeks
- Must have recovered to ≤ Grade 2 from all clinically significant toxicities related to prior therapies

The key exclusion criteria are as follows:
- Pathological findings consistent with small cell or any histology other than adenocarcinoma of the prostate. Minor (<20%) elements of neuroendocrine histology is acceptable.
- Diagnosed with other malignancies that are expected to alter life expectancy or interfere with disease assessment.
- At increased risk of hemorrhage or bleeding, or with a recent history (within last 6 months) of a thrombolytic event
- Has received prior treatment with mAB J591 or any other PSMA-targeted therapy
- Has known brain, liver, lytic bone, or lymph node metastases, ≥1cm in diameter
- Has a history of seizure and/or stroke within the past 6 months
- Has clinical or radiologic findings indicative of impending spinal cord compression
- Has evidence of a serious active or sub-clinical infection or angina pectoris, significantly prolonged QT interval, or other serious illness(es) involving the cardiac, respiratory, central nervous system, renal, hepatic, or hematological organ systems, that might impair the ability to complete this study or could interfere with determination of causality of any adverse effects experienced in this study, or which require treatment that could interact with study treatment, particularly with enzalutamide
- Has received treatment with any PARP inhibitors or with any platinum-based anti-neoplastic drugs
Presented by: A. Oliver Sartor, MD, Professor of Medicine, Urology, and Radiology, Director Radiopharmaceutical Trials, Mayo Clinic, Rochester, MN
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, May 31st – June 4th, 2024
Reference:

