(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago, IL between May 31st and June 4th, 2024 was host to a prostate, testicular, and penile cancers poster session.
Dr. Soumyajit Roy presented the results of a secondary analysis of the SPARTAN trial evaluating the effect of concomitant medications on treatment response and survival in non-metastatic castrate-resistant prostate cancer (nmCRPC).
Prior studies have demonstrated that exposure to common classes of concomitant medications, such as metformin and statins, influences survival in metastatic castrate-resistant prostate cancer patients receiving androgen receptor pathway inhibitors (ARPI).1 However, it is unclear whether such an association exists in nmCRPC patients receiving ARPIs. In this exploratory analysis of the SPARTAN trial, the investigators assessed whether receipt of commonly prescribed concomitant medications modulated the treatment effect of apalutamide on overall survival and metastasis-free survival in nmCRPC patients. Furthermore, they assessed whether receipt of these concomitant medications had any independent association with metastasis-free and overall survivals in the overall study cohort.
In the SPARTAN trial, men with nmCRPC who had a PSA doubling time ≤10 months were randomized to receive either ADT plus apalutamide or ADT plus placebo. 
Dr. Roy and colleagues focused on five commonly prescribed classes of medications:
- Biguanides (metformin)
- HMG-CoA reductase inhibitors (statins)
- Proton pump inhibitors (PPIs)
- Angiotensin-converting enzyme inhibitors (ACEIs)
- Acetylsalicylic acid (ASA)
The objectives of this exploratory analysis were to determine if:
- Receipt of any of these five classes of concomitant medications modulated the treatment effect of apalutamide on metastasis-free and overall survivals.
- Concomitant exposure to any of those medications had an independent association with metastasis-free and overall survivals.
To evaluate for a potential effect-modifying role for the concomitant medications, the investigators applied Cox regression models for overall and metastasis-free survivals with an interaction term between the medication class and the randomized treatment regimen, while controlling for potential confounders of this association. Confounders were chosen using data data-driven directed acyclic graph approach. They subsequently calculated inverse probability of treatment weighted (IPTW) Kaplan-Meier survival estimates for overall and metastasis-free survivals among patients stratified by concomitant receipt of each of the five different classes of medications. Given that five different classes of medications were being investigated, and to minimize the chance of a type I (i.e., false positive) error, a two-sided p-value of <0.01 was considered statistically significant.
The SPARTAN trial included a total of 1,207 patients randomized to either treatment arm. Outcomes and covariates data were available for 1,152 patients (placebo arm: n=380 patients; apalutamide arm: n=772). As demonstrated in the table below, only statins were an effect modifier of the treatment effect of apalutamide on metastasis-free survival (adjusted hazard ratio: 0.20 with statins versus 0.31 without statins). For overall survival, the investigators did not find any statistically significant heterogeneity of treatment effect from ADT plus apalutamide across subgroups stratified by concomitant exposure to the concomitant medications listed below.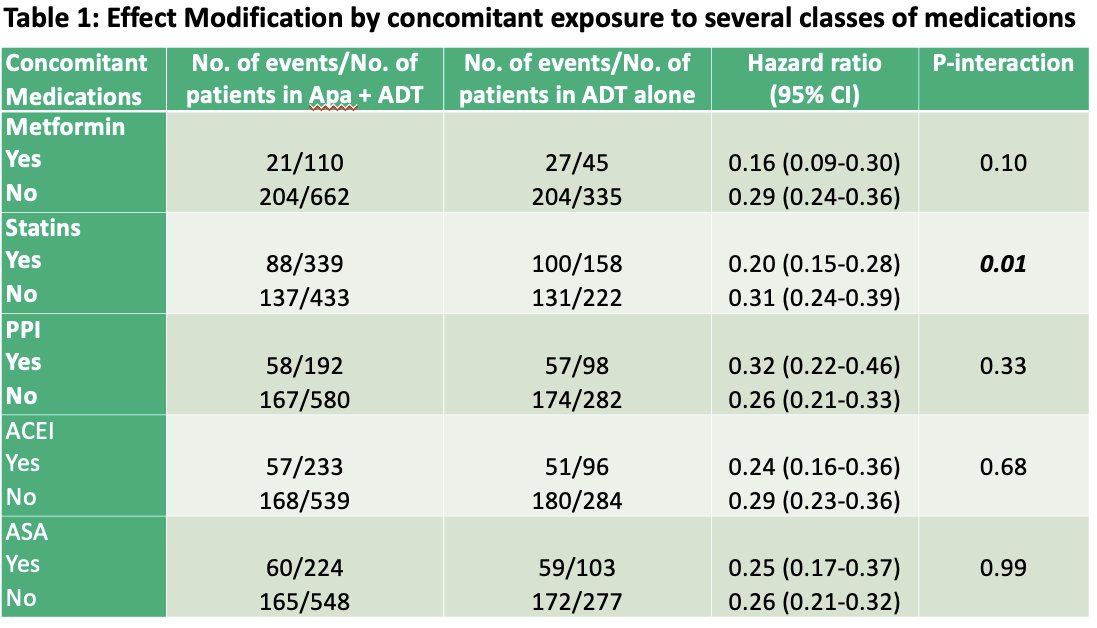
On IPTW-based analysis, patients receiving concomitant metformin (median: not reached vs 31 months; p=0.002) and concomitant ACEIs (median: 37 vs 29 months; p=0.006) had significantly superior metastasis-free survivals. Patients with exposure to the combination of metformin, ACEIs, and statins had significantly improved MFS (median: not reached vs 31 months; p<0.001).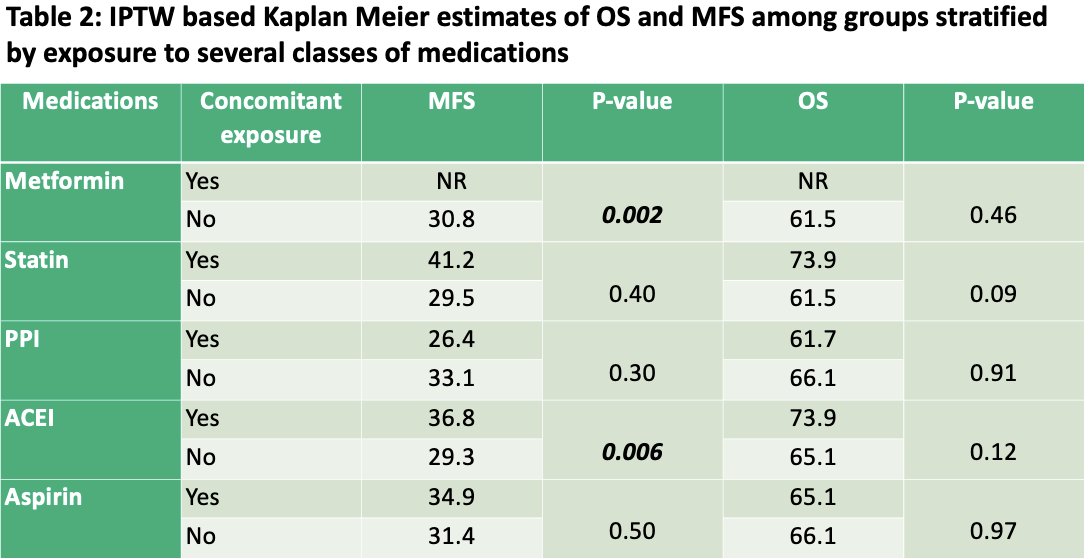
Dr. Roy and colleagues concluded that:
- In this exploratory analysis, treatment effects from apalutamide on metastasis-free and overall survivals were consistent across subgroups stratified by exposure to concomitant medications.
- Exposure to concomitant metformin or ACEI was associated with a significant improvement in metastasis-free survival/
Presented by: Soumyajit Roy, MBBS, MSc, Resident Physician, Department of Radiation Oncology, Rush University Medical Center, Chicago, IL
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, May 31st – June 4th, 2024
References:- Olokpa E, Mandape SN, Pratap S, Stewart LM V. Metformin regulates multiple signaling pathways within castration-resistant human prostate cancer cells. BMC Cancer 2022;22. https://doi.org/10.1186/S12885-022-10115-3.


