(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago, IL was host to a prostate, testicular, and penile cancers poster session. Dr. Alicia Morgans presented the results of a follow-up analysis of ARAMIS evaluating the association between PSA level <0.2 ng/ml and risk of radiological progression in patients with non-metastatic castration-resistant prostate cancer (nmCRPC).
Darolutamide is a structurally distinct and highly potent androgen receptor pathway inhibitor (ARPI) with low blood–brain barrier penetration and limited potential for clinically relevant drug–drug interactions. In the phase 3 ARAMIS trial, nmCRPC patients receiving darolutamide plus androgen-deprivation therapy (ADT) had significantly longer metastasis-free and overall survivals compared to patients receiving ADT alone.
In a prior analysis of the ARAMIS trial, metastatic progression was not consistently associated with prostate-specific antigen (PSA) progression, and pain progression was rare irrespective of metastatic and/or PSA progression. In this follow-up ad hoc analysis of the ARAMIS trial, Dr. Morgans reported prostate cancer-specific survival and patterns of disease progression for all patients from the ARAMIS trial and specifically for those patients who achieved serum PSA levels of <0.2 ng/mL.
In ARAMIS, patients with nmCRPC received darolutamide (n=955) or placebo (n=554), both with ADT. Using the primary data cut-off (September 3, 2018) excluding patients with baseline metastases, the investigators analyzed prostate cancer-specific survival accounting for other deaths as a competing risk, using metastasis-free survival as a data cutoff. Treatment groups-generated state sequence plots characterized patients by different events: radiological progression, PSA progression, or death. Based on PSA levels and conventional imaging every 16 weeks during ARAMIS, the cumulative incidence of time to radiological progression was compared between patients with PSA <0.2 ng/mL and those with PSA ≥0.2 ng/mL. In patients with PSA <0.2 ng/mL and who experienced radiological progression, they evaluated PSA levels at the time of radiological progression.
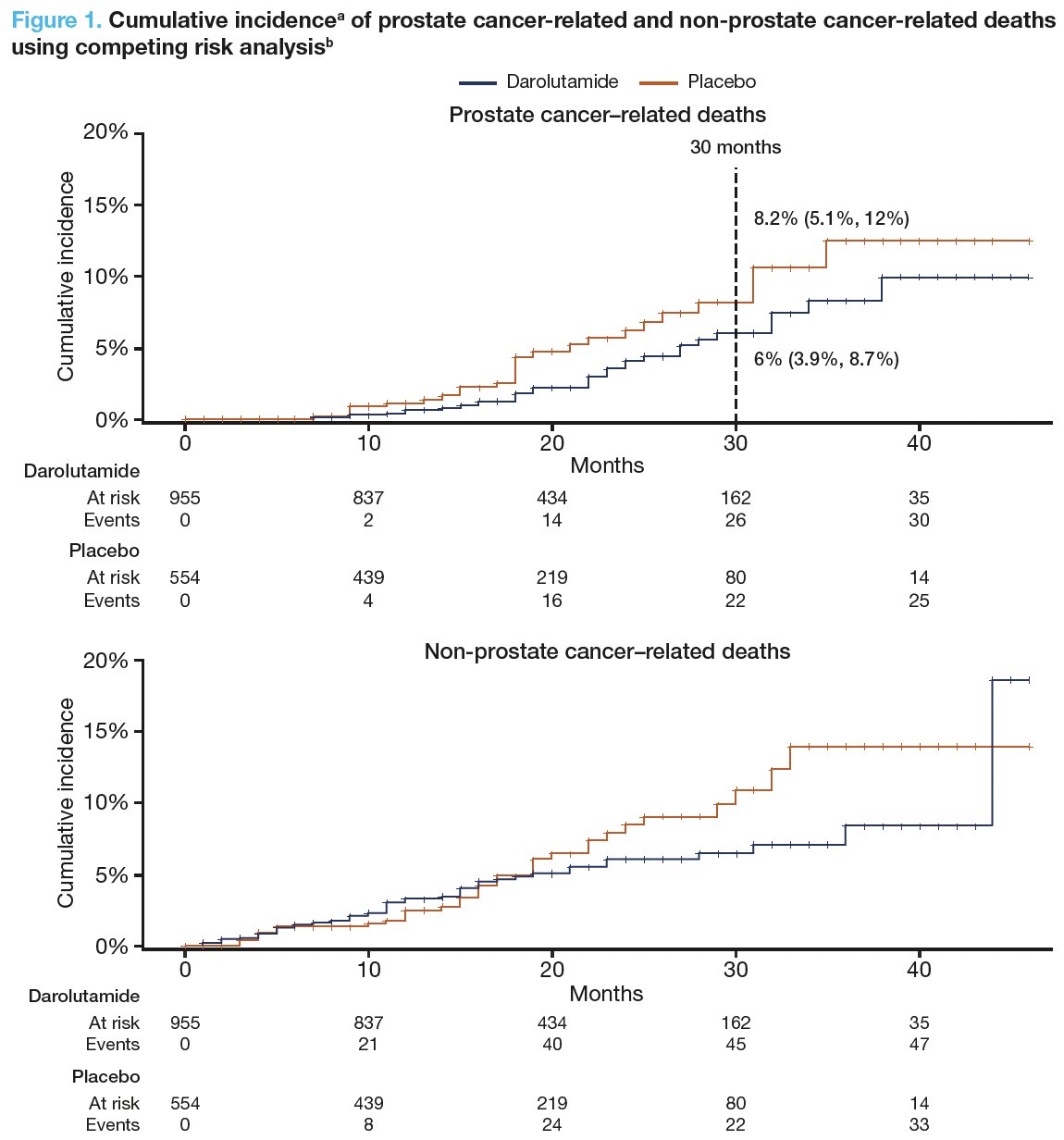
Darolutamide improved overall survival in ARAMIS, versus placebo (HR: 0.69; 95% CI: 0.53–0.88) and significantly reduced prostate cancer–related mortality (6% vs 8.2% at 30 months). Prostate cancer was the leading cause of death in patients with nmCRPC in both treatment arms (Figure 1).
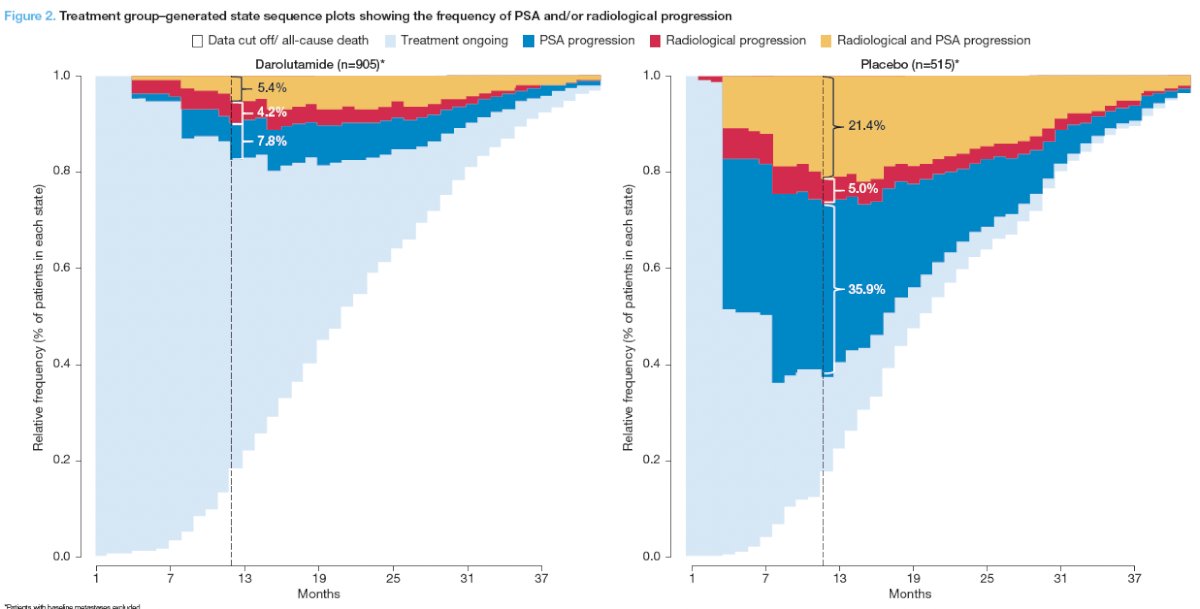
Fewer patients receiving darolutamide, compared to placebo, had PSA progression alone (7.8% versus 35.9%) or both PSA and radiological progression (5.4% versus 21.4%) at 12 months (Figure 2).
Among patients with PSA ≥0.2 ng/mL, those receiving darolutamide, versus placebo, had lower PSA levels at the time of radiological progression (median [95% CI], 2.4 [2.2–2.7] versus 3.7 [3.5–3.8] ng/mL). Darolutamide led to deep and durable PSA responses compared to placebo:
- Patients receiving darolutamide had a 50-fold higher chance of achieving PSA <0.2 ng/mL versus placebo (25.1% vs 0.5%)
- As previously reported for all patients in ARAMIS, darolutamide delayed time to PSA progression (median, 33.2 versus 7.3 months with placebo; HR 0.13; 95% CI: 0.11–0.16)
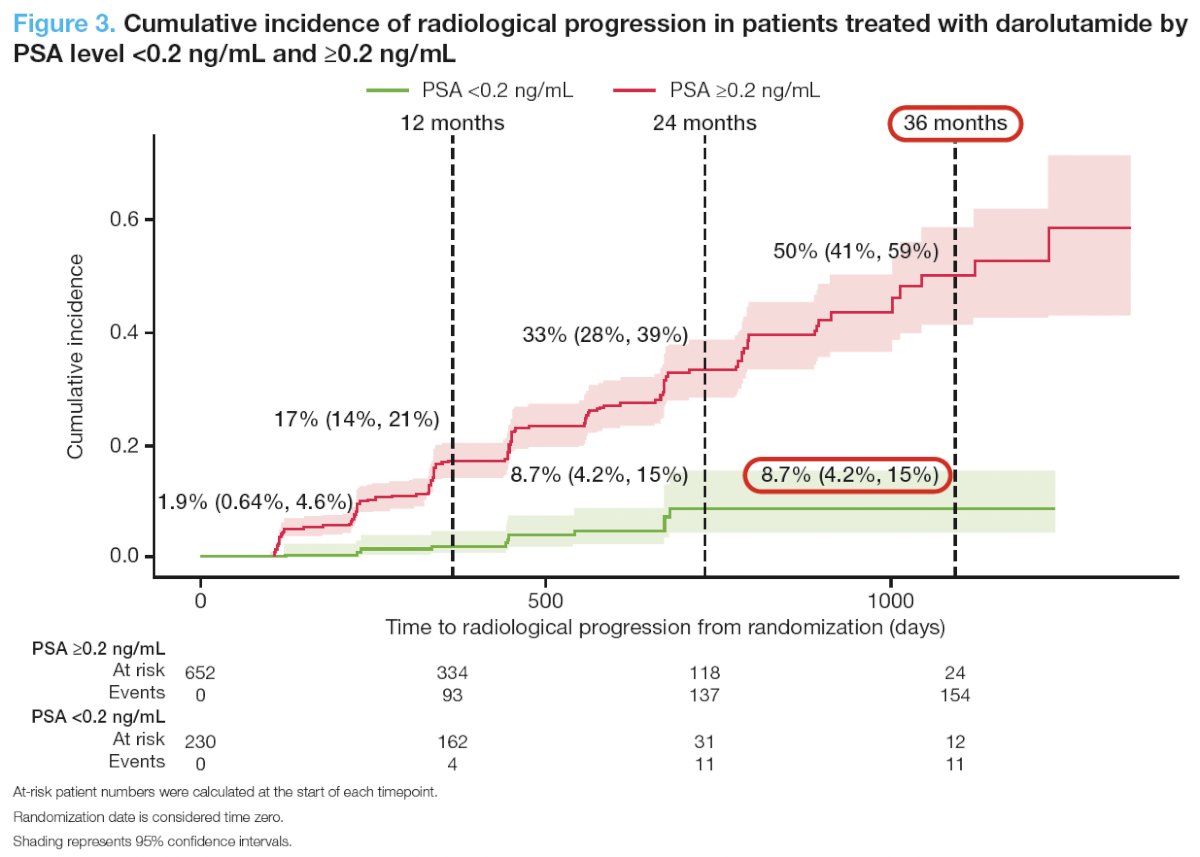
Darolutamide patients with PSA <0.2 ng/mL had a lower risk of radiological progression versus those with PSA ≥0.2 ng/mL, with rates of 8.7% versus 33% at 24 months. At 36 months, the cumulative incidence of radiological progression remained at 8.7% for darolutamide patients
with PSA <0.2 ng/mL and increased to 50% in patients with PSA ≥0.2 ng/mL. For the 11 patients with PSA <0.2 ng/mL and radiological progression, PSA levels at the time of radiological progression (range, 0.02 – 438.46 ng/mL) and time to radiological progression (4 – 22 months) did not follow any clear patterns.
Limitations to this analysis include the following:
- These post hoc analyses rely on descriptive graphics, involving correlations between post-baseline outcomes and limited follow-up time to further evaluate additional progression events
- Radiological progressions were captured based on per-protocol scans, not reflecting real-world practice; PSA assessments were not mandatory after patients had radiological progression
- The data are hypothesis-generating and need to be further confirmed
Dr. Morgans concluded her presentation as follows:
- In ARAMIS, darolutamide reduced the risk of death with an HR of 0.69 vs placebo; moreover, darolutamide reduced prostate cancer–specific mortality.
- Findings from this post hoc analysis reaffirm the dominant role of prostate cancer in the mortality landscape of patients with nmCRPC.
- Fewer patients receiving darolutamide experienced PSA progression with or without radiological progression.
- Darolutamide was also associated with deep and durable PSA responses compared with placebo.
- In the darolutamide arm, patients who achieved a PSA <0.2 ng/mL, had a low risk of radiological progression over 24 months that did not increase through the end of the study
- Radiological progression can occur in the absence of PSA progression and with low PSA values, and thus regular imaging is still recommended in this nmCRPC setting
- Further research is needed on the association between low PSA values (<0.2 ng/mL) and radiological progression.
Presented by: Alicia K. Morgans, MD, MPH, Associate Professor, Department of Medicine, Medical Director of the Survivorship Program at Dana-Farber Cancer Institute, Massachusetts General Hospital, Boston, MA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
References:
- Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic castration-resistant prostate cancer. N Engl J Med. 2019;380(13):1235-1246.
- Fizazi K, Shore N, Tammela TL, et al. Nonmetastatic, Castration-Resistant Prostate Cancer and Survival with Darolutamide. N Engl J Med. 2020 Sep 10;383(11):1040-1049.


