(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago, IL was host to a prostate, testicular, and penile cancers trials in progress poster session. Dr. Karim Fizazi presented the phase 3 MK-5684-004 study comparing MK-5684, a CYP11A1 inhibitor, to a next-generation hormonal agent (NHA) switch in patients with metastatic castration-resistant prostate cancer (mCRPC) progressing after one prior NHA.
Activating androgen receptor (AR) somatic mutations are a common mechanism of resistance to AR-directed therapies in mCRPC, and they may enable continued hormonal dependence. Upstream targeting of androgen biosynthesis may provide a therapeutic advantage over available AR antagonist therapies for mCRPC. Opevesostat (MK-5684; ODM-208) is an oral nonsteroidal inhibitor of cytochrome P450 11A1 (CYP11A1), which catalyzes the first and rate-limiting step of steroid biosynthesis. Inhibition of CYP11A1 by opevesostat can potentially suppress the production of all steroid hormones and precursors that may promiscuously activate the AR signaling pathway. In the phase 1/2 CYPIDES trial, opevesostat showed anti-tumor activity in patients with heavily pretreated mCRPC, especially in those with AR ligand-binding domain (AR-LBD) mutations.1 The randomized, open-label, phase 3 MK-5684-004 study (NCT06136650) will evaluate the efficacy and safety of opevesostat versus NHA switch in patients with molecularly unselected mCRPC after one prior NHA treatment.
The study design is summarized below. This study will randomize 1,500 mCRPC patients unselected for AR-LBD mutations with progressive disease during or after one NHA for hormone-sensitive prostate cancer (either metastatic or non-metastatic) or non-metastatic 1:1 to either:
- Arm 1: Opevesostat 5 mg orally twice daily + dexamethasone 1.5 mg and fludrocortisone 0.1 mg orally once daily
- Arm 2: NHA switch
- Abiraterone acetate 1,000 mg orally once daily + prednisone 5 mg orally twice daily or enzalutamide 160 mg orally once daily
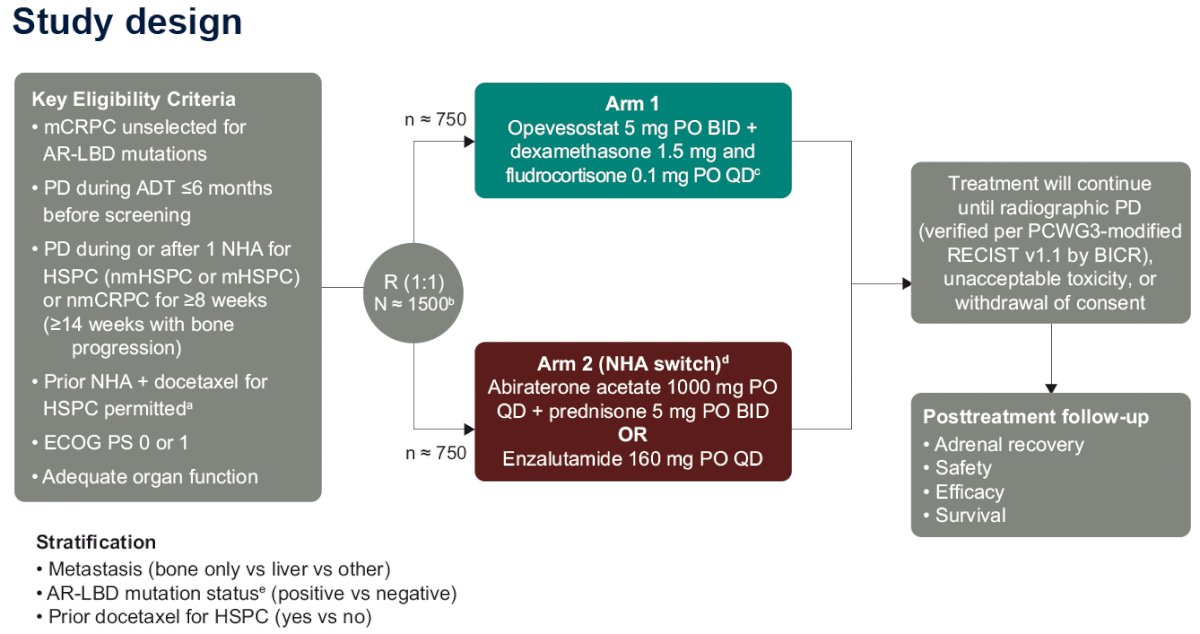
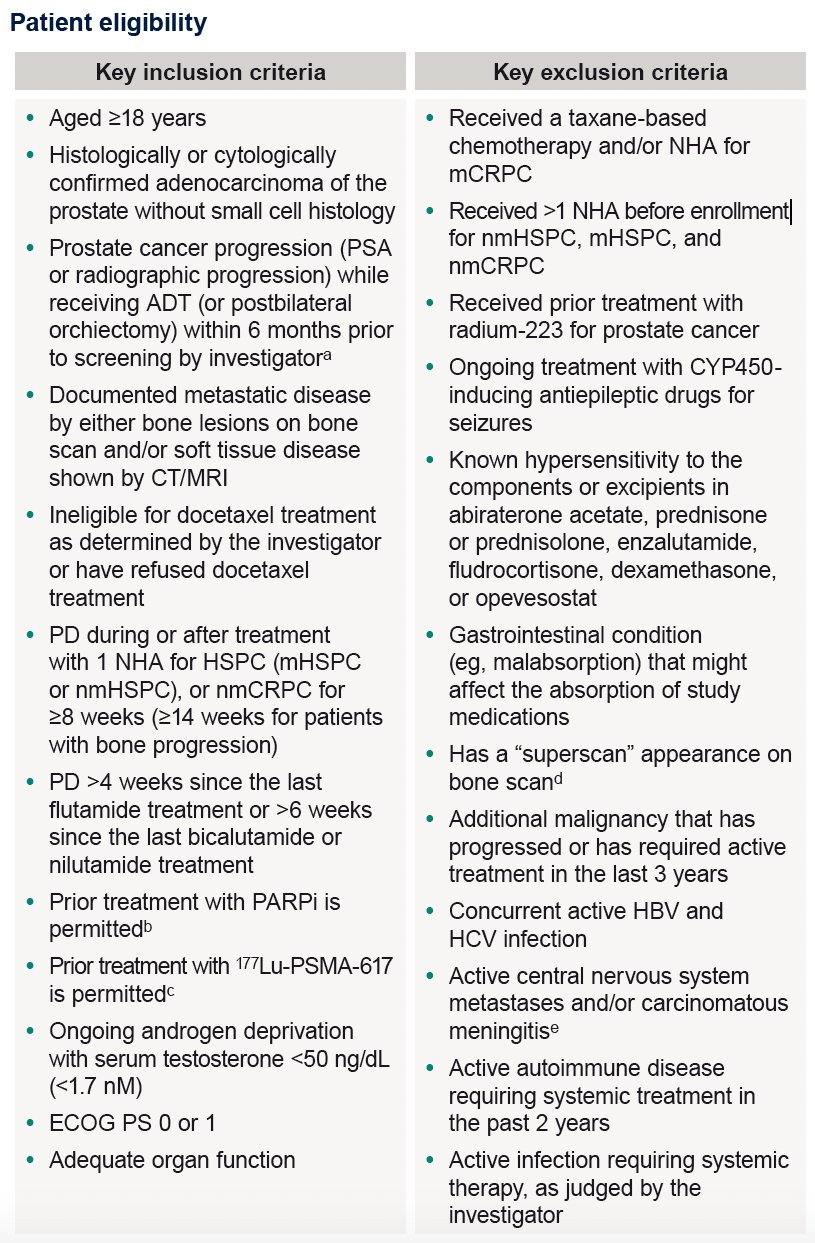
The key eligibility criteria are summarized in the table below:
The primary study objective is to evaluate the following for opevesostat versus abiraterone acetate or enzalutamide in patients with AR-LBD mutation–positive and –negative mCRPC, separately:
- Radiographic progression-free survival (rPFS) per Prostate Cancer Clinical Trials Working Group 3 (PCWG3)–modified RECIST v1.1 by blinded independent central review (BICR)
- Overall survival (OS)
The secondary outcomes are as follows:
- Time from randomization to initiation of the first subsequent anticancer therapy or death (TFST)
- Objective response rate (ORR) and duration of response (DOR) per PCWG3-modified RECIST v1.1 by BICR in patients with measurable disease
- Time to pain progression (TTPP; time from randomization to pain progression as determined by item 3 of the Brief Pain Inventory–Short Form [BPI-SF] and by the Analgesic Quantification Algorithm score)
- Change from baseline, time to deterioration (TTD), and overall improvement in the total score of the Functional Assessment of Cancer Therapy–General (FACT-G) subscale of the Functional Assessment of Prostate Cancer (FACT-P) questionnaire
- Time to prostate-specific antigen (PSA) progression (time from randomization to the date of PSA progression)
- PSA response rate (≥50% reduction from baseline in the PSA level measured twice ≥3 weeks apart)
- Time to first symptomatic skeletal-related event (SSE; time from randomization to the first occurrence of an SSE)
- Safety and tolerability
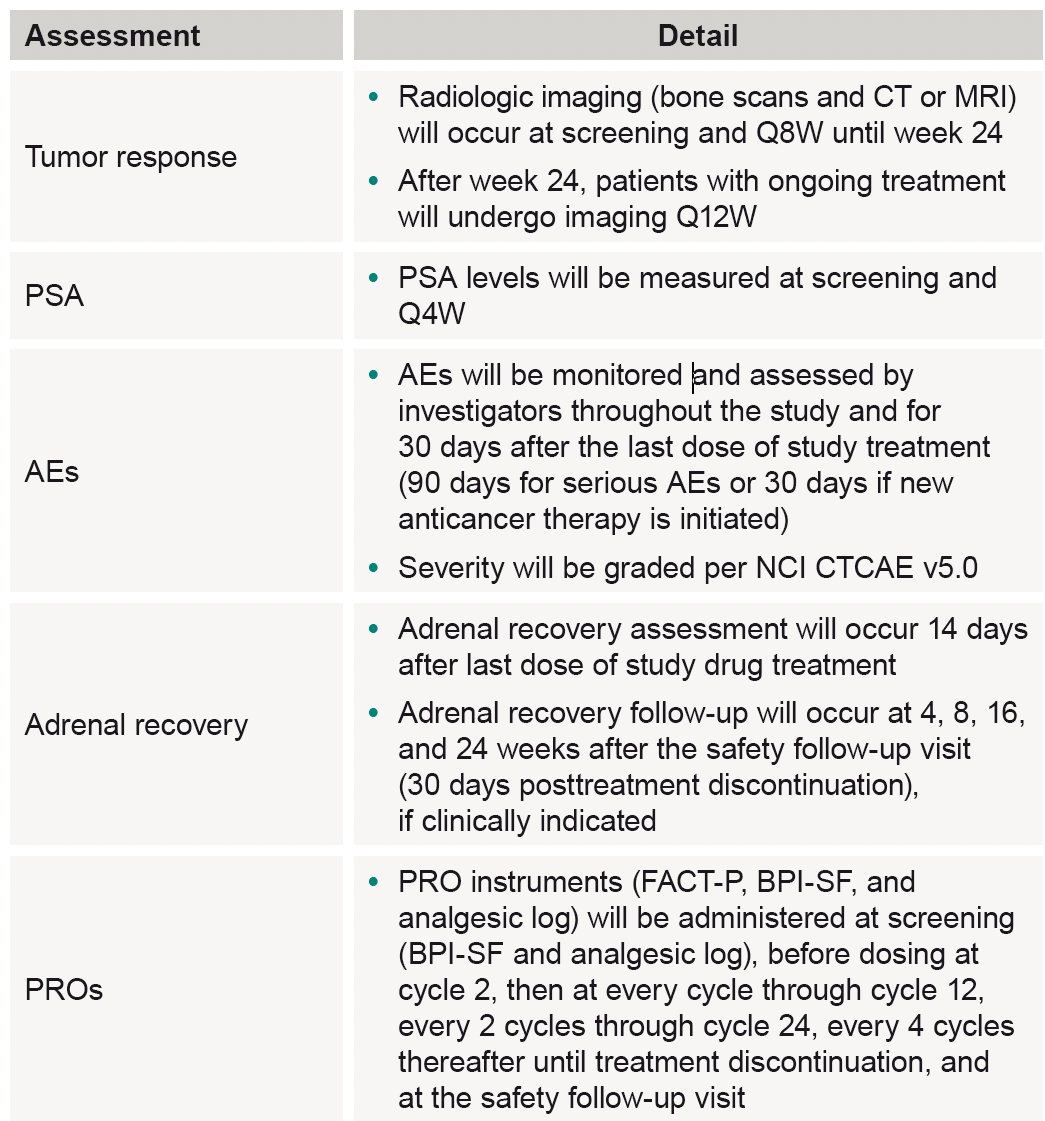
The study assessments and follow-up will be performed as follows:
The current study status is as follows:
Presented by: Karim Fizazi, MD, PhD, Professor, Department of Medicine, Institut Gustave Roussy, Paris, France
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
Reference:


