(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago, IL between May 31st and June 4th, 2024 was host to a prostate, testicular, and penile cancers trials in progress poster session. Dr. Atish Choudhury presented A-DREAM/Alliance A032101, an ongoing phase II trial of ADT interruption in metastatic hormone-sensitive prostate cancer (mHSPC) patients responding exceptionally to an androgen receptor pathway inhibitor (ARPI).
Systemic therapy intensification with ARPI addition to ADT has been demonstrated to improve overall survival outcomes, compared to ADT alone, in numerous pivotal phase 3 trials.1-5 However, it remains unclear whether continuous treatment is requisite for optimal cancer control in all patients. Defining which patients might benefit from a time-delimited course of treatment (i.e. treatment de-intensification) is of significant interest. Hormonal therapy intensification with ARPI + ADT is associated with short- and long-term toxicities, as well as decrements in quality-of-life outcomes.
Favorable PSA declines have been associated with prolonged overall survival in clinical trials of ADT + ARPIs. The investigators hypothesized that treatment interruption in a subset of patients who achieve exceptional response to upfront therapy, including a novel ARPI, will allow them to remain treatment-free with testosterone recovery for a prolonged period afterwards, leading to improved quality-of-life outcomes and reduced treatment/financial toxicity.
The study design is illustrated below. mHSPC patients receiving ADT + ARPI who achieve a serum PSA level <0.2 ng/ml (stable/decreasing) following 18–24 months of ADT and ≥12 months of an ARPI will have both ADT and ARPI discontinued. Serum PSA and testosterone levels will be re-evaluated every three months, with scans and quality-of-life assessments performed every six months (every three months if PSA is rising). Triggers for treatment re-initiation include the occurrence of either:
- PSA ≥5 ng/ml
- Radiographic changes
- Prostate cancer-related symptoms
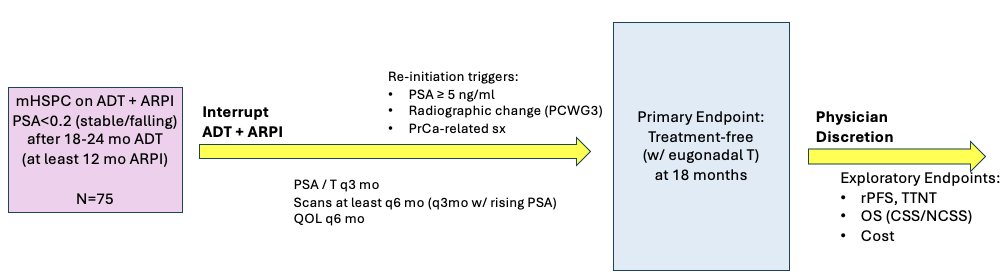
The primary study outcome is the 18 months of eugonadal treatment-free survival. Secondary outcomes include:
- Time to eugonodal testosterone (i.e, >150 ng/dL)
- Duration off therapy
- Changes in quality of life (change in FACT-P total score from baseline to 24 months after treatment interruption, change in FACT-P subscales from baseline to 24 months, and change in total score and subscales from baseline to 6, 12, and 18 months after treatment interruption)
- rPFS
- Time to next therapy
- Overall survival
- Cancer-specific and non-cancer-specific survivals
- Estimate of cost-savings (total drug cost, out-of-pocket costs to patient)
- Correlation of tissue and blood-based biomarkers with clinical endpoints
The key eligibility criteria are as follows:
- Evidence of metastatic disease by conventional imaging prior to starting intense ADT
- On testosterone suppression for mHSPC for 540–750 days without breaks; on ARPI for ≥ 360 days with breaks up to 60 days allowed.
- Prior intermittent ADT, prior local therapy, prior radiotherapy to metastatic sites permitted.
- No prior surgical castration, no ARPI prior to mHSPC diagnosis, and no experimental treatment for mHSPC.
- Age ≥ 18 years, ECOG 0-2
- PSA ≥ 5 ng/ml and testosterone ≥ 150 ng/dl (or not known to have been hypogonadal) prior to starting intense ADT
- PSA < 0.2 ng/ml (stable or falling for 3 consecutive measurements) and testosterone < 50 ng/dl prior to enrollment.
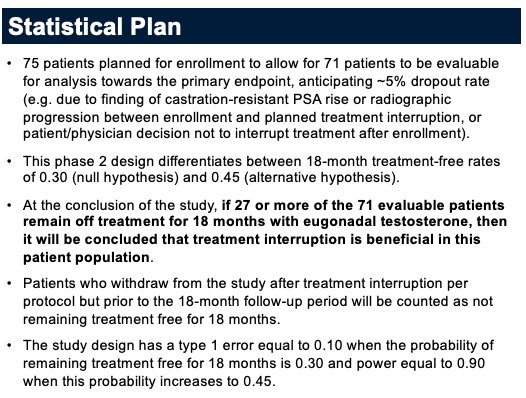
The study statistical plan is summarized below:
The study (NCT05241860) was activated through Alliance across all NCTN sites in July 2022 and completed enrollment in March 2024. 79 total (78 eligible) of planned 75 patients have been enrolled, with follow-up pending. The baseline patient characteristics are summarized below. The median serum PSA prior to initiating systemic therapy was 19 ng/ml. The majority of patients (78%) have baseline M1b disease. One-third of patients have CHAARTED high-volume disease. The most commonly utilized ARPI at study entry is abiraterone acetate (63%), followed by enzalutamide (16%) and darolutamide (15%). Notably, 31% of patients received radiation to metastatic sites.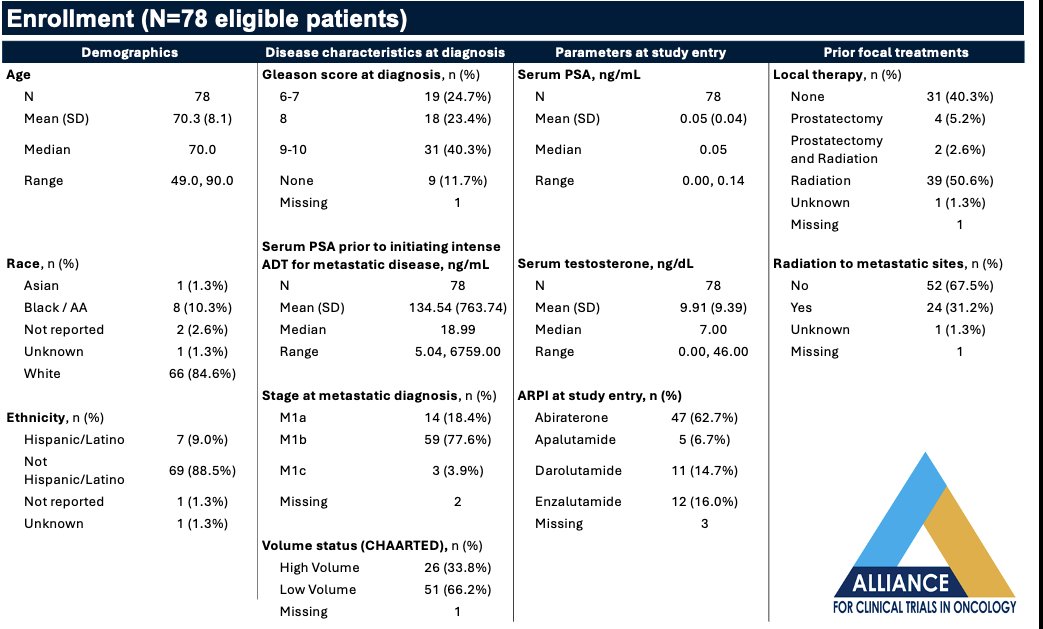
Presented by: Atish Dipankar Choudhury, MD, PhD, Assistant Professor of Medicine, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, May 31st – June 4th, 2024
References:
- James ND, de Bono JS, Spears MR, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med. 2017;377(4):338-351.
- Fizazi K, Tran N, Fein L, et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med. 2017;377(4):352-360.
- Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med 2019 Jul 11;381(2):121-131.
- Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy with Enzalutamide or Placebo in Men with Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol. 2019 Nov 10;37(32):2974-2986.
- Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med 2019 Jul 4;381(1):13-24.


