(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago, IL between May 31st and June 4th, 2024 was host to a prostate, testicular, and penile cancers poster session. Dr. Arun Azad presented the results of an ad hoc analysis of TALAPRO-2 evaluating the utility of ctDNA burden as a prognostic biomarker of efficacy outcomes in metastatic castrate-resistant prostate cancer patients (mCRPC) randomized to talazoparib plus enzalutamide versus placebo plus enzalutamide.
TALAPRO-2 (NCT03395197) demonstrated that first-line treatment with talazoparib plus enzalutamide significantly improved radiographic progression-free survival (rPFS) compared to placebo plus enzalutamide for mCRPC patients unselected for homologous recombination repair gene alterations.1 Circulating tumor DNA (ctDNA) burden is a candidate prognostic biomarker for treatment response and resistance with potentially broad utility across treatments and tumor types. In this study, Dr. Azad and colleagues evaluated the prognostic value of baseline ctDNA burden and changes in ctDNA burden at Week 9 in the TALAPRO-2 cohort.
They retrospectively analyzed serial ctDNA samples from patients at baseline and at Week 9 using FoundationOne® Liquid CDx (Foundation Medicine, Cambridge, MA, USA). Plasma tumor fraction was calculated based on aneuploidy using a prototype assay, with ctDNA burden categorized as high (ctDNA burden quantifiable) versus low (unknown ctDNA burden). The data cutoff date was August 16, 2022.
In the all-comers, intent-to-treat population, 678 patients were evaluated for ctDNA burden at baseline:
- 26% (89/337) of talazoparib plus enzalutamide patients had a high ctDNA burden; 74% (248/337) had a low ctDNA burden
- 29% (98/341) of placebo plus enzalutamide patients had a high ctDNA burden; 71% (243/341) had a low ctDNA burden
High ctDNA burden at baseline was prognostic of inferior rPFS in both the talazoparib plus enzalutamide and placebo plus enzalutamide arms (Table 1).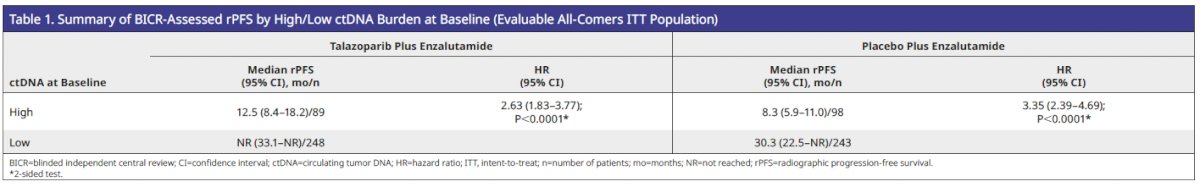
A relatively favorable median rPFS was observed for patients with low ctDNA at baseline and at Week 9 for talazoparib plus enzalutamide (n=206; Figure 1) and placebo plus enzalutamide (n=207; Figure 2).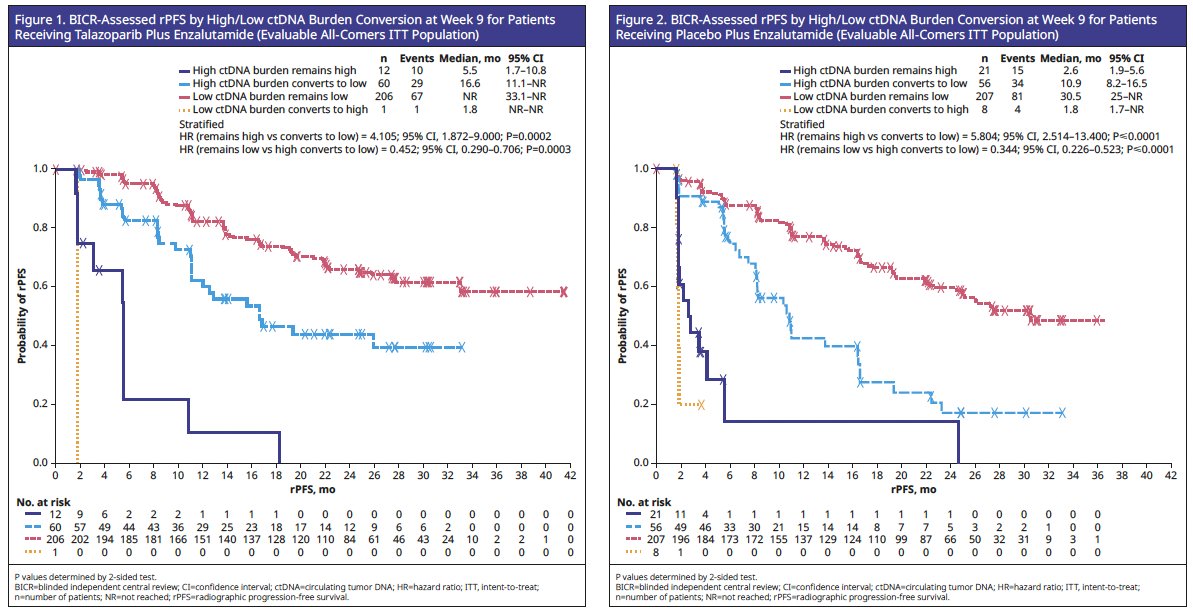
At Week 9, 72 patients in the talazoparib plus enzalutamide arm and 77 patients in the placebo plus enzalutamide arm were evaluated for ctDNA conversion from high to low. In both treatment arms, conversion from high to low ctDNA was prognostic of improved rPFS, compared to patients who remained ctDNA-high:
- Talazoparib plus enzalutamide arm: Median rPFS was 16.6 months for patients who had ctDNA conversion from high to low versus 5.5 months for patients who remained ctDNA-high.
- Placebo plus enzalutamide arm: Median rPFS was 10.9 months for patients who had ctDNA conversion from high to low versus 2.6 months for patients who remained ctDNA-high.
Patients in both treatment arms who remained ctDNA-low had a more favorable rPFS compared to those converting from high to low ctDNA:
- Talazoparib plus enzalutamide: hazard ratio (HR)=0.45; 95% confidence interval (CI): 0.29–0.71; p=0.0003
- Placebo plus enzalutamide: HR=0.34; 95% CI: 0.23–0.52; p<0.0001
Dr. Azad concluded that:
- High ctDNA burden at baseline was adversely prognostic for rPFS, and ctDNA conversion from high to low at Week 9 was prognostic of improved rPFS in TALAPRO-2
- These results support the broad prognostic utility of ctDNA burden in mCRPC patients receiving enzalutamide +/- talazoparib
- Limitations to this study included that not all clinical trial sites were able to perform ctDNA collection and most samples were below the limit of quantification using this prototype tumor fraction algorithm
- Future analyses will include use of an enhanced tumor fraction algorithm with a higher sensitivity
Presented by: Arun Azad, PhD, MBBS, FRACP, Associate Professor, Department of Medicine, Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, May 31st – June 4th, 2024
References:

