(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago, IL was host to a prostate, testicular, and penile cancers poster session. Dr. Karim Fizazi presented a novel non-negative matrix factorization-based homologous recombination deficiency score and its subsequent exploration in TALAPRO-2, a phase III study of talazoparib plus enzalutamide versus placebo plus enzalutamide as first-line treatment in metastatic castration-resistant prostate cancer (mCRPC) patients.
In TALAPRO-2 (NCT03395197), ‘all-comer’ mCRPC patients treated in the 1st line setting with talazoparib plus enzalutamide had superior radiographic progression-free survival (rPFS), compared to patients receiving placebo plus enzalutamide.1 In this study, Dr. Fizazi and colleagues developed and evaluated a novel non-negative matrix factorization (NMF)-based homologous recombination deficiency (HRD) score and used it to explore potential associations of HRD with efficacy in the TALAPRO-2 population.
A previously published composite HRD score incorporating genomic loss of heterozygosity, large-scale transitions, and telomeric allelic imbalances was used as the “original” HRD reference score.2 Two datasets were used to generate the HRD score for TALAPRO-2:
- A FoundationOne®Liquid CDx (F1LCDx) dataset of prospectively collected/retrospectively analyzed screening plasma samples (n=681)
- A baseline/archival tumor transcriptomic dataset generated via an oncology biomarker panel (with 10 additional genes implicated in poly[ADP-ribose] polymerase [PARP] inhibitor sensitivity; n=304)
The Cancer Genome Atlas Prostate Adenocarcinoma (TCGA-PRAD) dataset was used to train a novel NMF-based HRD predictive score incorporating gene expression (assessed by RNA
sequencing) and homologous recombination repair (HRR) 12 genomic features (Figures 1 and 2).
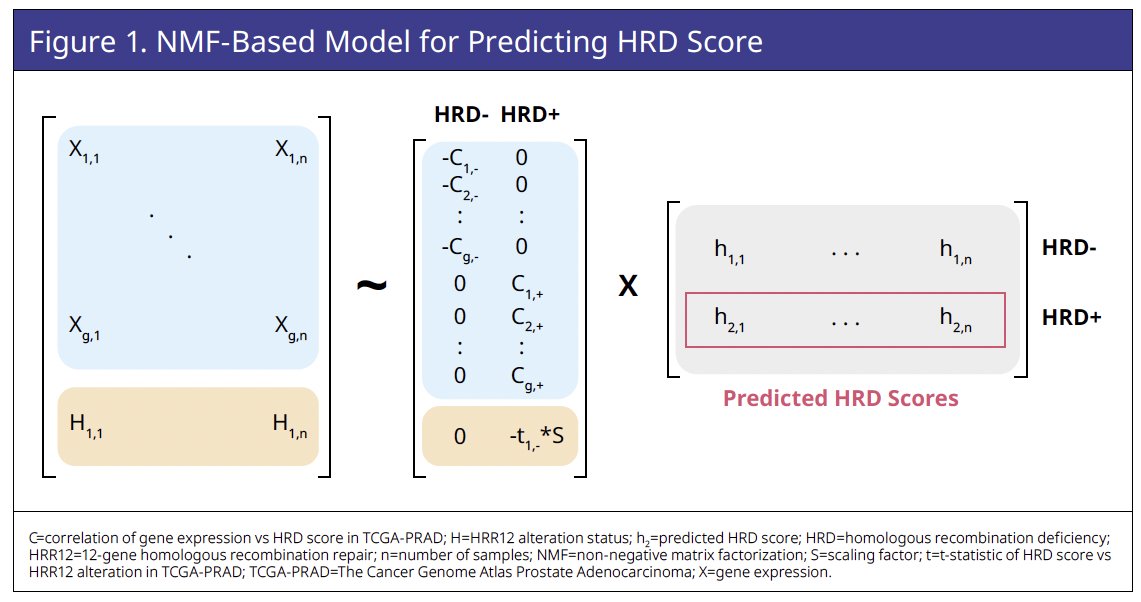
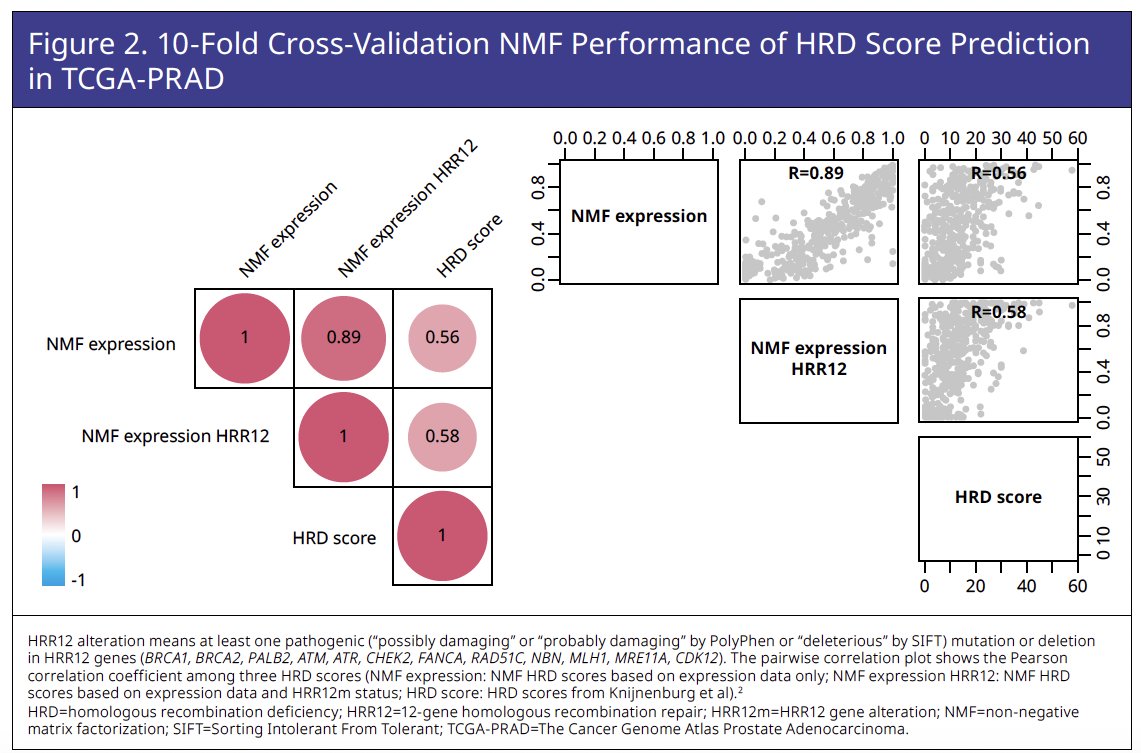
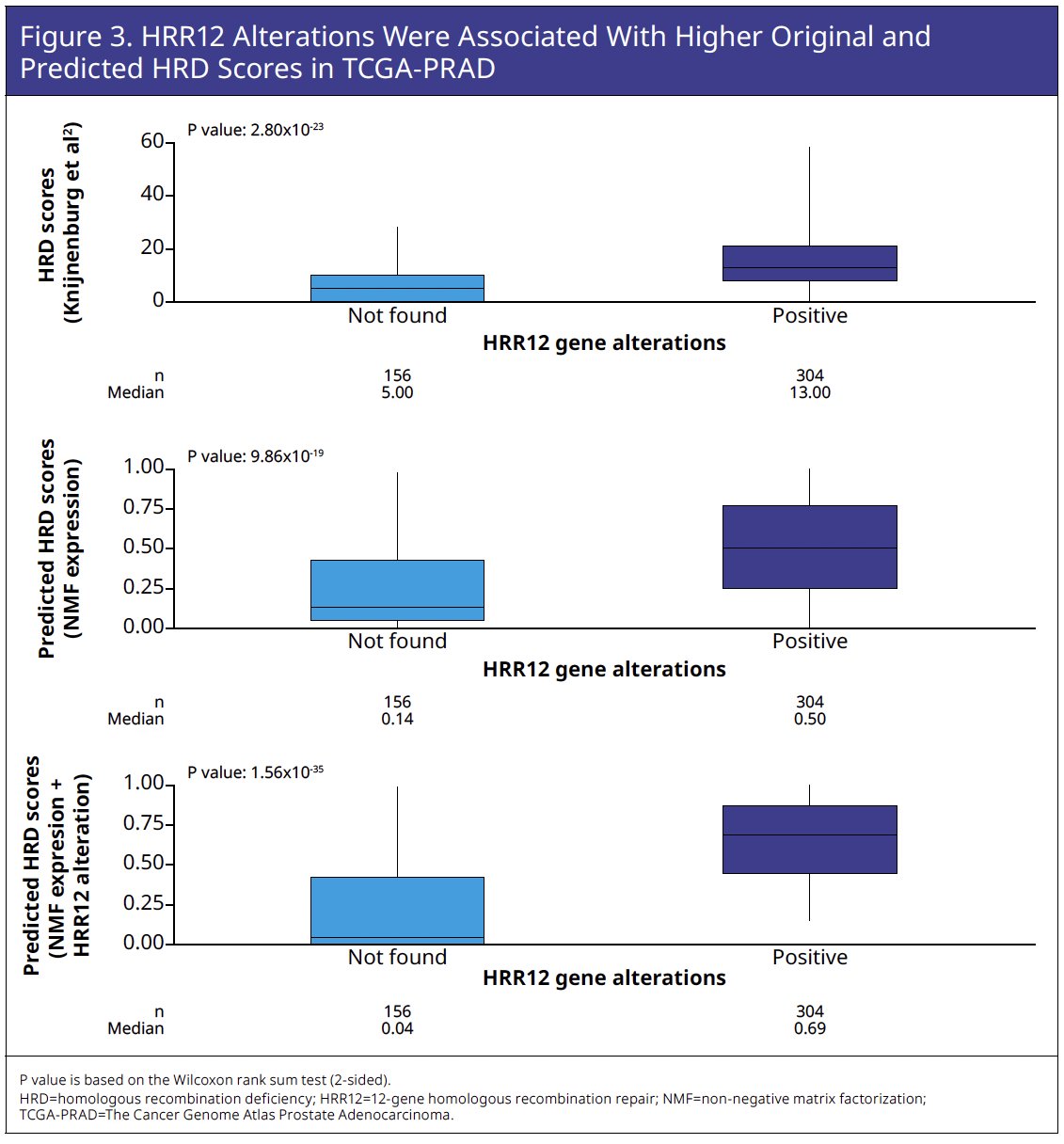
HRR12 genomic features in TCGA-PRAD were associated with higher original and predicted HRD scores (Figure 3).
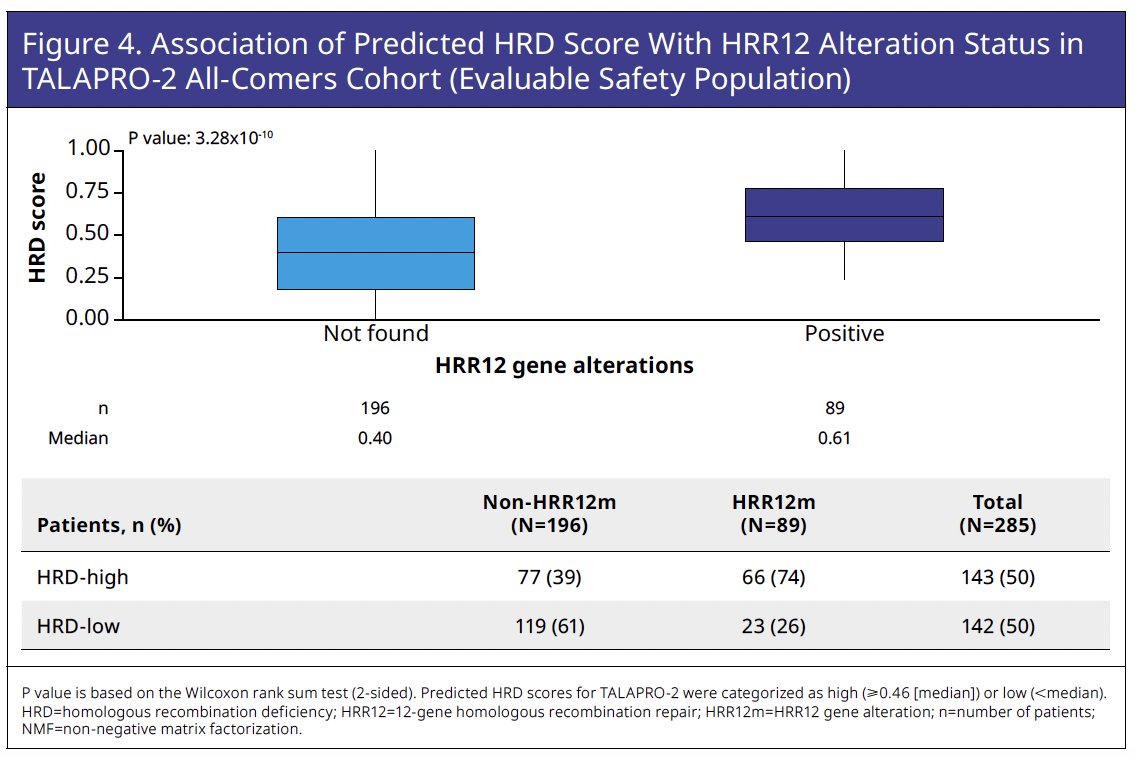
Although predicted HRD score is primarily based on gene expression, it proved significantly associated with HRR12 gene alteration (HRR12m) status based on F1LCDx variant calling of retrospectively sequenced circulating tumor DNA (ctDNA; Wilcoxon rank sum test
p=3.28x10-10). Of 89 patients with HRR12m by F1LCDx, 66 patients (74%) were HRD-high and 23 patients (26%) were HRD-low. Conversely, of 196 patients who were non HRR12m by F1LCDx, 77 patients (39%) were HRD-high and 119 patients (61%) were HRD low, suggesting the presence of a subgroup of patients who had HRD in the absence of detected HRR12m (Figure 4).
rPFS was numerically shorter for HRD-high versus HRD-low within each treatment arm, although this difference was not statistically significant (p>0.05). More favorable rPFS was demonstrated for talazoparib plus enzalutamide compared with placebo plus enzalutamide in both patients with HRD-high (hazard ratio [HR]=0.49, p=0.004) and HRD-low (HR=0.48, p=0.006) scores (Figure 5).
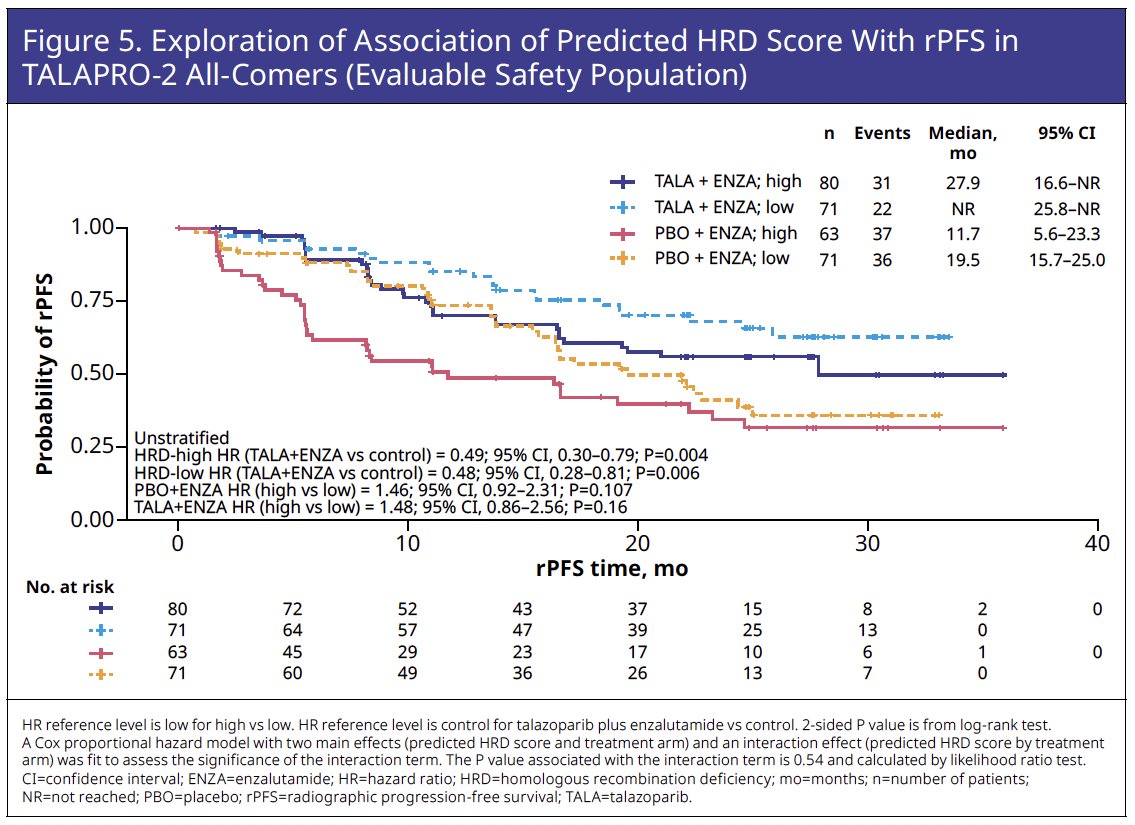
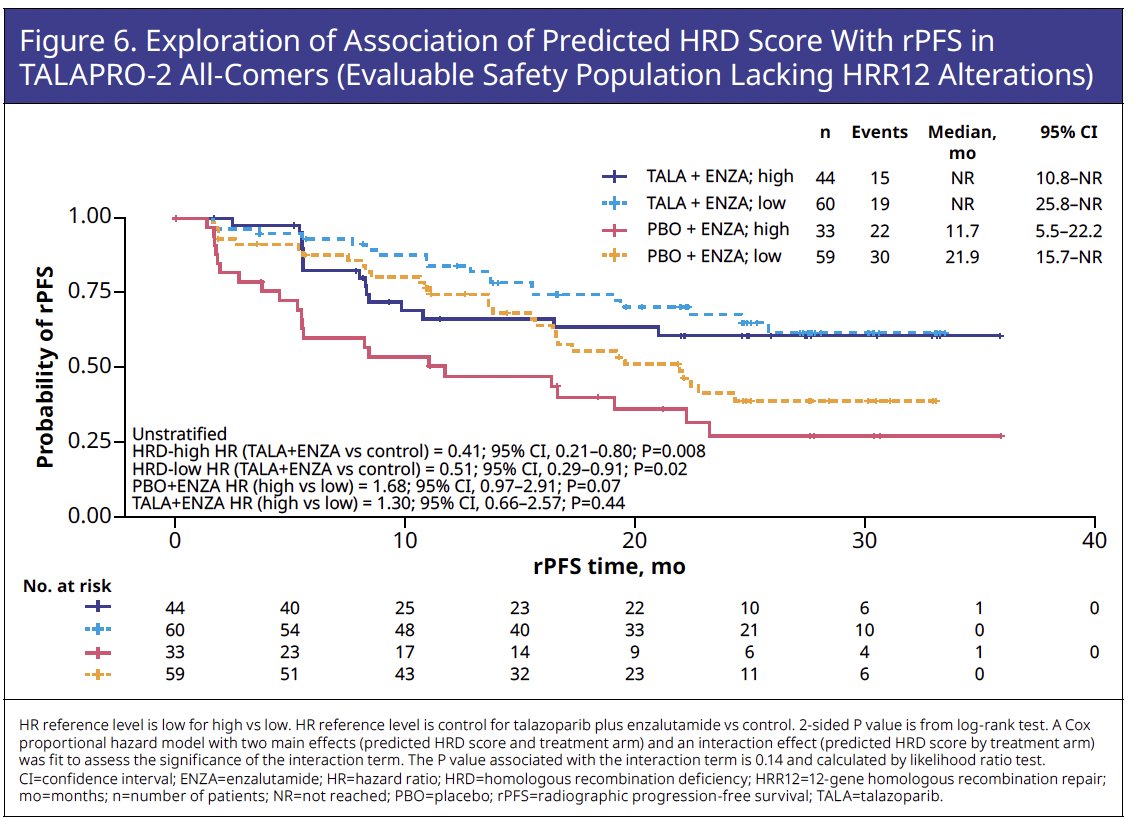
These trends of numerically, but not statistically significant, shorter rPFS in patients with an HRD-high versus HRD-low score and comparable rPFS benefit favoring talazoparib plus enzalutamide over placebo plus enzalutamide in patients with an HRD-high and HRD-low scores were evident in the subgroup of patients lacking HRR12m (Figure 6).
Dr. Fizazi concluded:
- This exploratory analysis of TCGA-PRAD resulted in discovery of a novel HRD score, incorporating both gene expression and HRR12 genomic attributes.
- In the evaluable TALAPRO-2 all-comers safety population, this HRD score was associated with ctDNA HRR12m status
- Within each treatment arm, rPFS survival was numerically shorter for patients with an HRD-high versus HRD-low score, although this difference was not statistically significant (p>0.05).
- Patients with an HRD-high and HRD-low score exhibited comparable rPFS benefit in terms of hazard ratio favoring talazoparib plus enzalutamide over placebo plus enzalutamide.
- These trends of numerically, but not statistically significant, shorter rPFS in patients with an HRD-high versus HRD-low score and comparable rPFS benefit favoring talazoparib plus enzalutamide over placebo plus enzalutamide in patients with an HRD-high and HRD-low score were evident in the subgroup of patients lacking HRR12m.
Presented by: Karim Fizazi, MD, PhD, Professor, Department of Medicine, Institut Gustave Roussy, Paris, France
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
References:
- Agarwal N, Azad AA, Carles J, et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): A randomized, placebo-controlled, phase 3 trial. Lancet. 2023;402(10398): 291-303.
- Knijnenburg TA, Wang L, Zimmermann MT, et al. Genomic and Molecular Landscape of DNA Damage Repair Deficiency across The Cancer Genome Atlas. Cell Rep. 2018;23(1): 239-254.e6.


