(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured a session on prostate cancer, and a presentation by Dr. Luke Nordquist discussing results of COBRA, an assessment of the safety and efficacy of 64Cu-SAR-bisPSMA in patients with biochemical recurrence of prostate cancer following definitive therapy.
Between 20-40% of patients with prostate cancer will relapse within 10 years of their primary prostate cancer treatment, as identified by increasing PSA levels. Most relapses will occur within 5 years after definitive therapy, thus early diagnosis of biochemical recurrence with accurate staging is essential to inform the best treatment strategy. Over the last several years, PSMA has been used as an imaging target in prostate cancer.
64Cu-SAR-bisPSMA may offer several advantages over the currently approved PSMA PET agents due to the bivalent structure of SAR-bisPSMA and longer half-life (t1/2) of 64Cu (12.7 hours), compared to the monovalent agents utilizing 18F and 68Ga (t1/2 < 2 hours):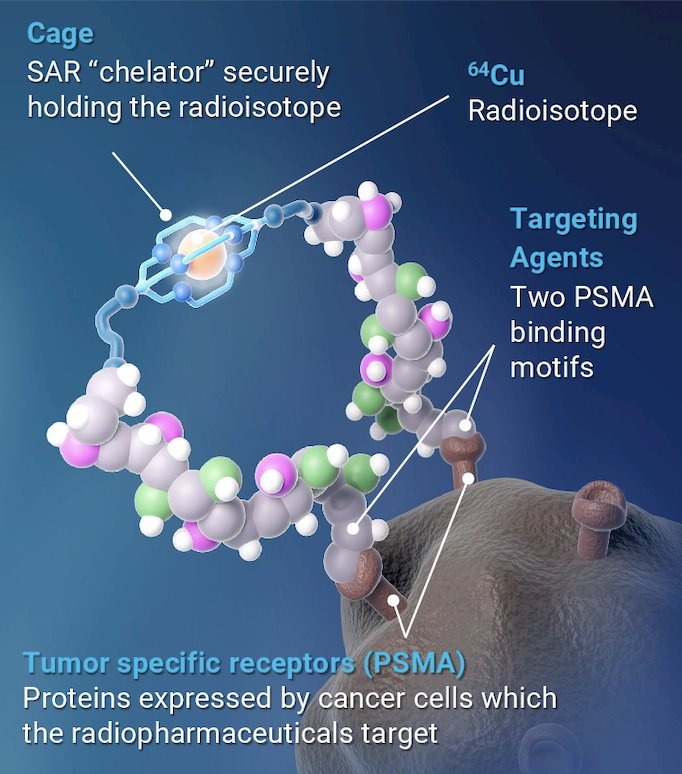
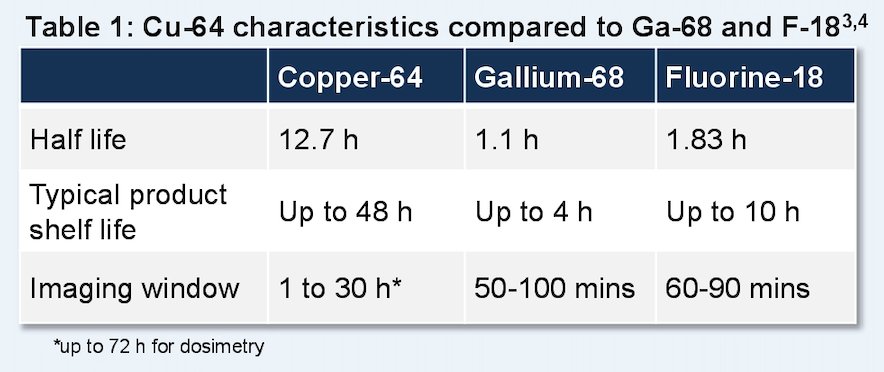
Clinical/translational evidence has demonstrated higher tumor uptake (2-3x), prolonged retent, ion, and detection of additional prostate cancer lesions using 64Cu-SAR-bisPSMA compared to approved PSMA agents.
The COBRA study was a phase I/II study assessing the safety and efficacy of 64Cu-SAR-bisPSMA (200 MBq) in prostate cancer patients with biochemical recurrence and negative or equivocal standard of care imaging. Patients underwent PET/CT on Day 0 and Day 1 (1-4 hours and 24 ± 6 hours post-dose, respectively), interpreted by 3 blinded central readers. The study design is as follows: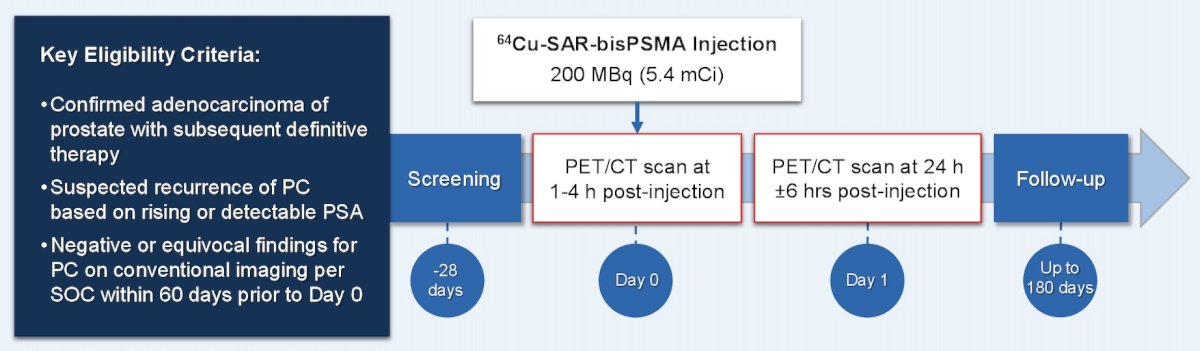
The PET/CT results were assessed against a reference standard (histopathology, standard of care imaging, PSA response) that was determined by an independent, blinded, central expert panel. Efficacy endpoints included detection rate and positive predictive value. Additionally, the intended change in prostate cancer treatment due to the 64Cu-SAR-bisPSMA results was recorded.
There were 52 patients that received 64Cu-SAR-bisPSMA (safety set), two replacements (protocol deviations), 50 patients that proceeded to follow-up, 8 patients without a reference standard, and 42 patients with a reference standard (efficacy set). Only one adverse event was related to 64Cu-SAR-bisPSMA (Grade 2 worsening of type II diabetes, resolved). The total number of lesions identified increased from Day 0 (70) to day 1 (129) across three readers, representing an 82% increase in detection: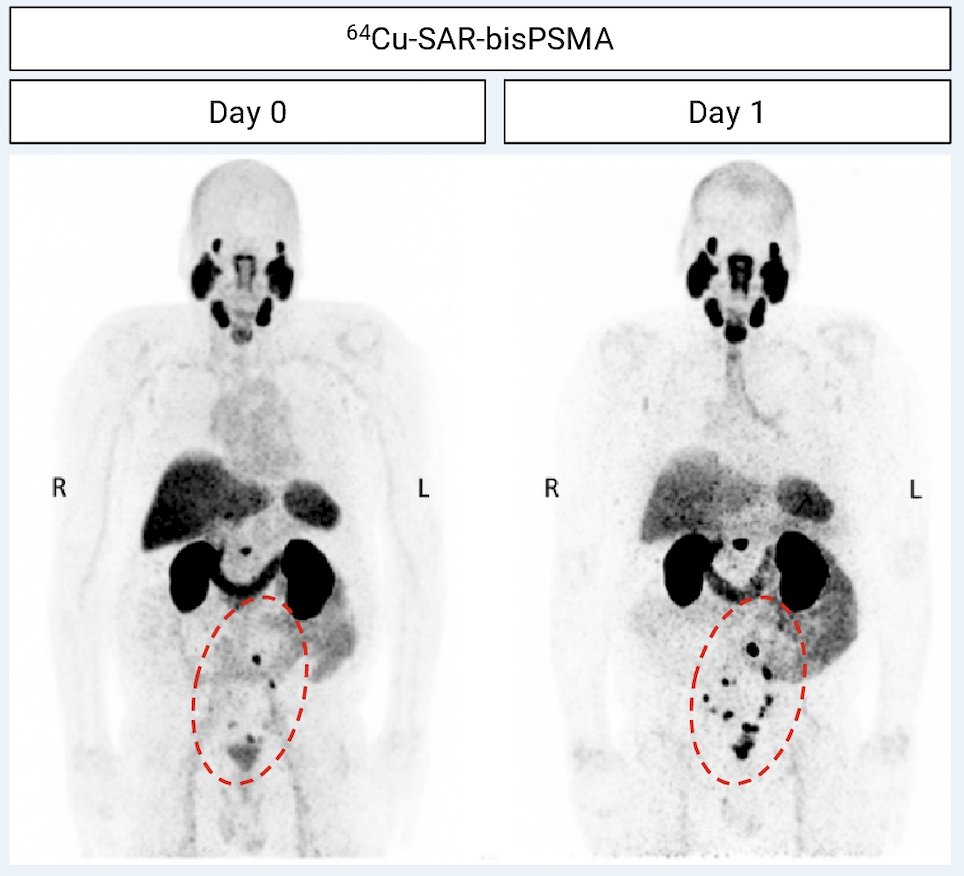
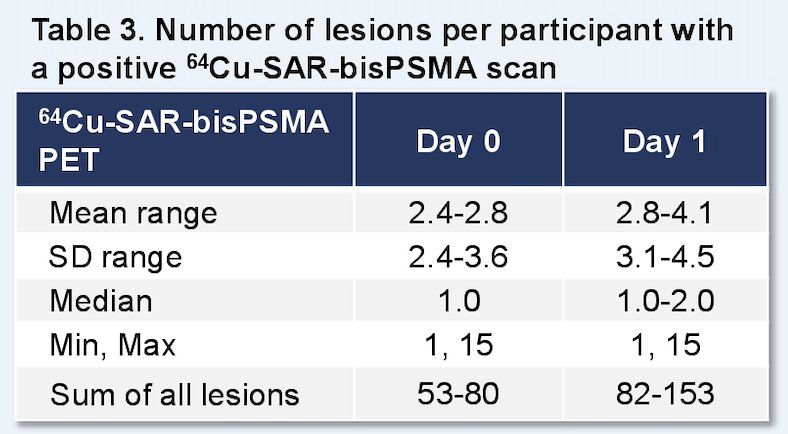
The Day 0 detection rate range across the readers was 44–58% (95% CI 30.0–71.8), increasing on Day 1 to 58–80% (95% CI 43.2–90.0), representing a 34% increase in patients having a positive 64Cu-SAR-bisPSMA scan on Day 1 (71%) versus Day 0 (53%) across the average of 3 readers. Pelvic lymph nodes had a Day 0 positive predictive value range of 71.4–87.5% (95% CI 29.0–99.7) and Day 1 of 50.0–61.5% (95% CI 15.7–86.1). The relative decrease in Day 1 positive predictive value was related to the challenges to obtain the reference standard for additional lesions identified on Day 1, where biopsy of all lesions was not feasible and due to the low sensitivity of current standard of care imaging that were used for co-localization: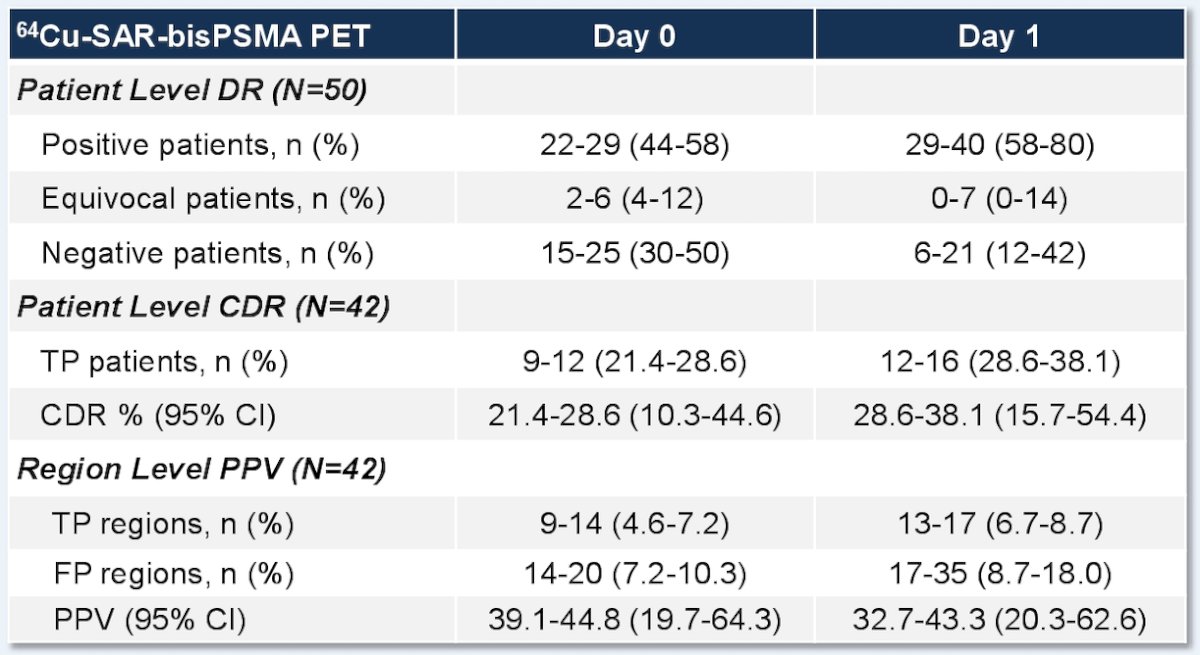
Of note, 64Cu-SAR-bisPSMA detects lesions in the 2 mm range: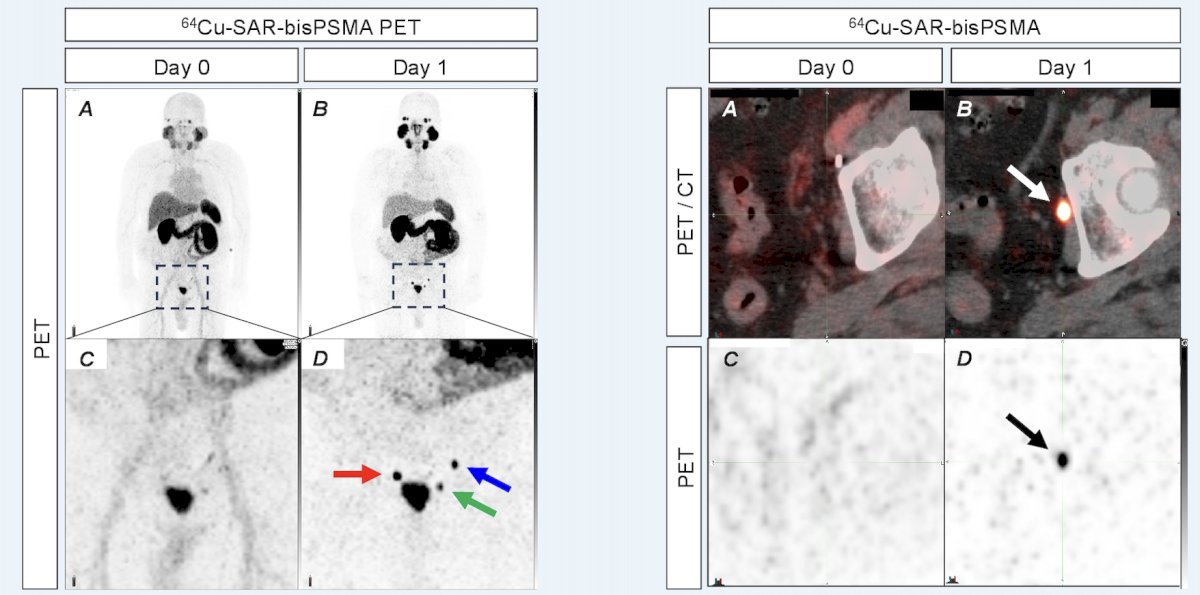
Importantly, the 64Cu-SAR-bisPSMA imaging led to clinicians changing their intended treatment plans in 48% of patients.
Dr. Nordquist concluded his presentation discussing results of COBRA, an assessment of the safety and efficacy of 64Cu-SAR-bisPSMA in patients with biochemical recurrence of prostate cancer following definitive therapy with the following take home messages:
- COBRA showed that 64Cu-SAR-bisPSMA is safe and effective in detecting prostate cancer lesions in patients with biochemical recurrence
- Only one treatment emergent adverse event was related to 64Cu-SAR-bisPSMA, which resolved
- In patients with a negative or equivocal standard of care scan, 64Cu-SAR-bisPSMA identified lesions (as small as 2 mm) in up to 80% of patients
- More lesions and more patients with a positive scan were identified on next-day imaging, a feature that currently approved PSMA tracers cannot offer
- PET results led to clinicians changing the intended treatment plan in approximately half of the patients
- Taken altogether, these findings have important clinical implications as the identification of lesions in biochemical recurrence patients can inform different treatment pathways for patient biochemical recurrence
Presented by: Luke Nordquist, MD, FACP, Chief Executive Officer, XCancer Omaha, Omaha, NE
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
Related Content: COBRA Trial Shows Promise for 64Cu-SAR-bisPSMA PET in Biochemical Recurrence - Neal Shore


