(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31 and June 4 was host to the Poster Session: Genitourinary Cancer: Prostate, Testicular, and Penile. Dr. Benjamin Maughan presented a retrospective observational cohort study using ConcertAI to assess rapid and deep prostate-specific antigen (PSA) response to apalutamide plus ADT and survival in metastatic castration-sensitive prostate cancer (mCSPC) in real-world practice in the US (OASIS Project).
Dr. Maughan commenced his presentation by discussing that Apalutamide, combined with androgen deprivation therapy (ADT), proves to be an effective life-prolonging treatment option for mCSPC, as demonstrated in the TITAN trial.1 Additionally, Apalutamide+ADT has shown efficacy in eliciting early and profound PSA responses. These early and substantial reductions in PSA levels have been associated with extended survival and improved clinical outcomes in TITAN patients with mCSPCand those receiving Abiraterone for metastatic castration-resistant disease.2,3 The primary objective of this study was to evaluate the correlation between PSA levels and long-term clinical outcomes in adults with mCSPC treated with upfront Apalutamide+ADT in real-world practice.
This retrospective observational cohort study utilized ConcertAI, a platform integrating data from electronic health records of over 4 million patients from medical oncology clinics across the United States (U.S.). ConcertAI Patient 360 captures staging, PSA values, and castration resistance status. ConcertAI, an AI-powered SaaS data company in healthcare, is increasingly utilized as a medical research tool in oncology.
For this analysis, patients aged 18 years and older diagnosed with mCSPC between January 2018 and September 2022, who initiated treatment with Apalutamide+ADT and were followed up for at least 6 months, were included. The investigators examined correlations between time to 50% and 90% declines in PSA values (PSA50 and PSA90), time to undetectable PSA (≤0.2 ng/mL), and 24-month overall survival (OS). They employed multivariate Cox proportional hazard models adjusted for age, comorbidities, BMI, and baseline PSA to estimate Adjusted Hazard Ratios (aHRs) for OS.
They included in this analysis 183 patients with mCSPC who initiated Apalutamide+ADT treatment and had monthly PSA testing. The clinical and demographic characteristics are outlined in the table below. Briefly, the mean age was 73 years, median baseline PSA 4.8 ng/mL (IQR 0.8, 20.7), and the mean duration of follow-up was 18 months.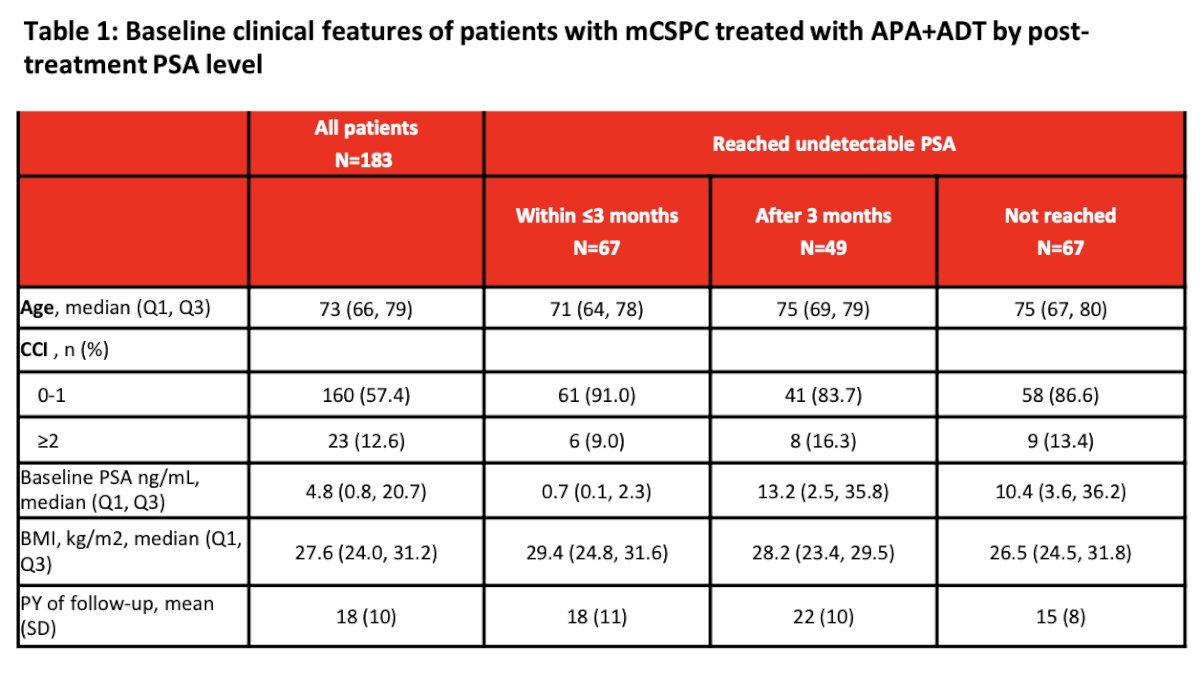
The majority of patients (68%) experienced a PSA reduction of at least 50% within 3 months after initiating treatment, with 37% reaching an undetectable PSA level (≤0.2 ng/mL). The median time to achieving an undetectable PSA was 2.1 (1.1, 3.0) months in the group with responses within 3 months and 4.7 (3.0, 24.6) months in the group with responses after 3 months. Dr. Maughan noted that survival rates increase not only when PSA responses occur more rapidly (within ≤3 months) but also when they achieve deep PSA reductions (undetectable vs. PSA50).
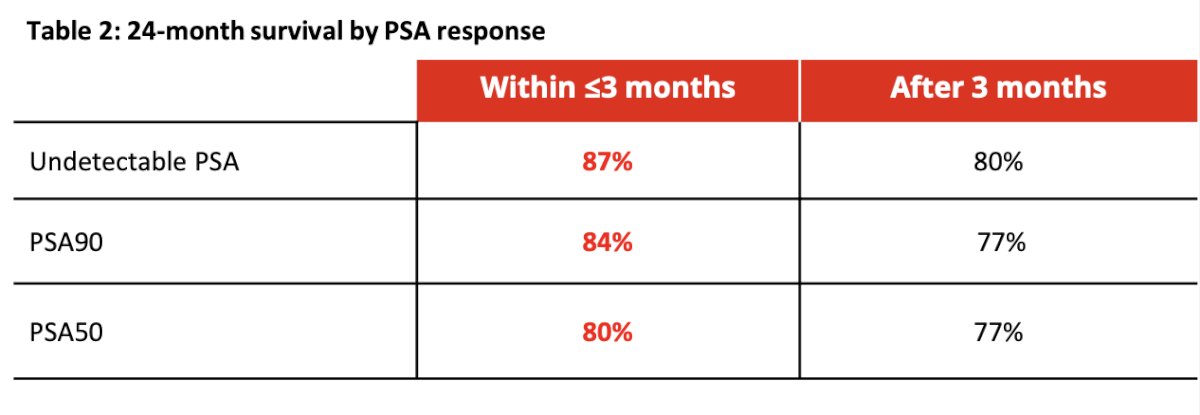
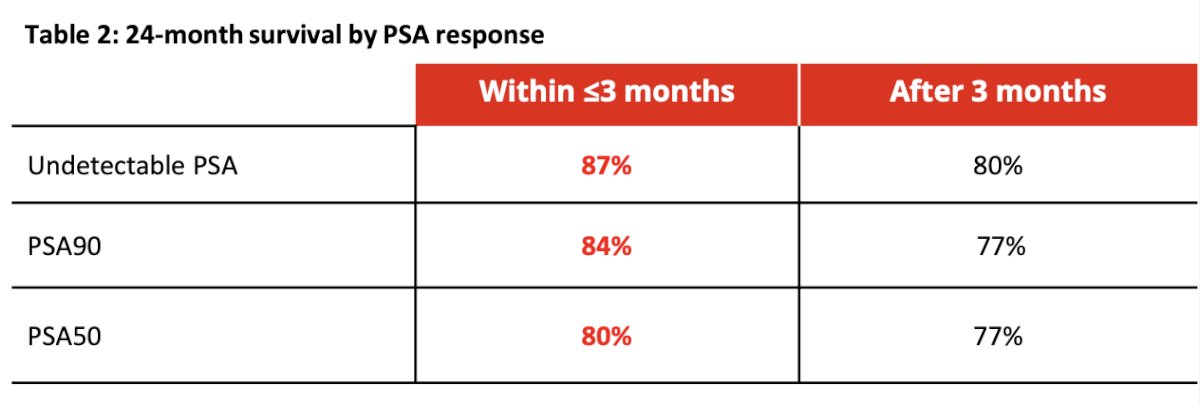
The Kaplan-Meier curves below depict overall survival (OS) stratified by time to undetectable PSA and time to PSA50 response, categorized as ≤3 months or >3 months. The adjusted hazard ratio (aHR) for OS when an undetectable PSA was achieved within ≤3 months was 0.15 (95% CI 0.05-0.47, p<0.001). Similarly, the aHR for OS was 0.27 (95% CI 0.12-0.65, p<0.001) when the undetectable PSA response was achieved after >3 months.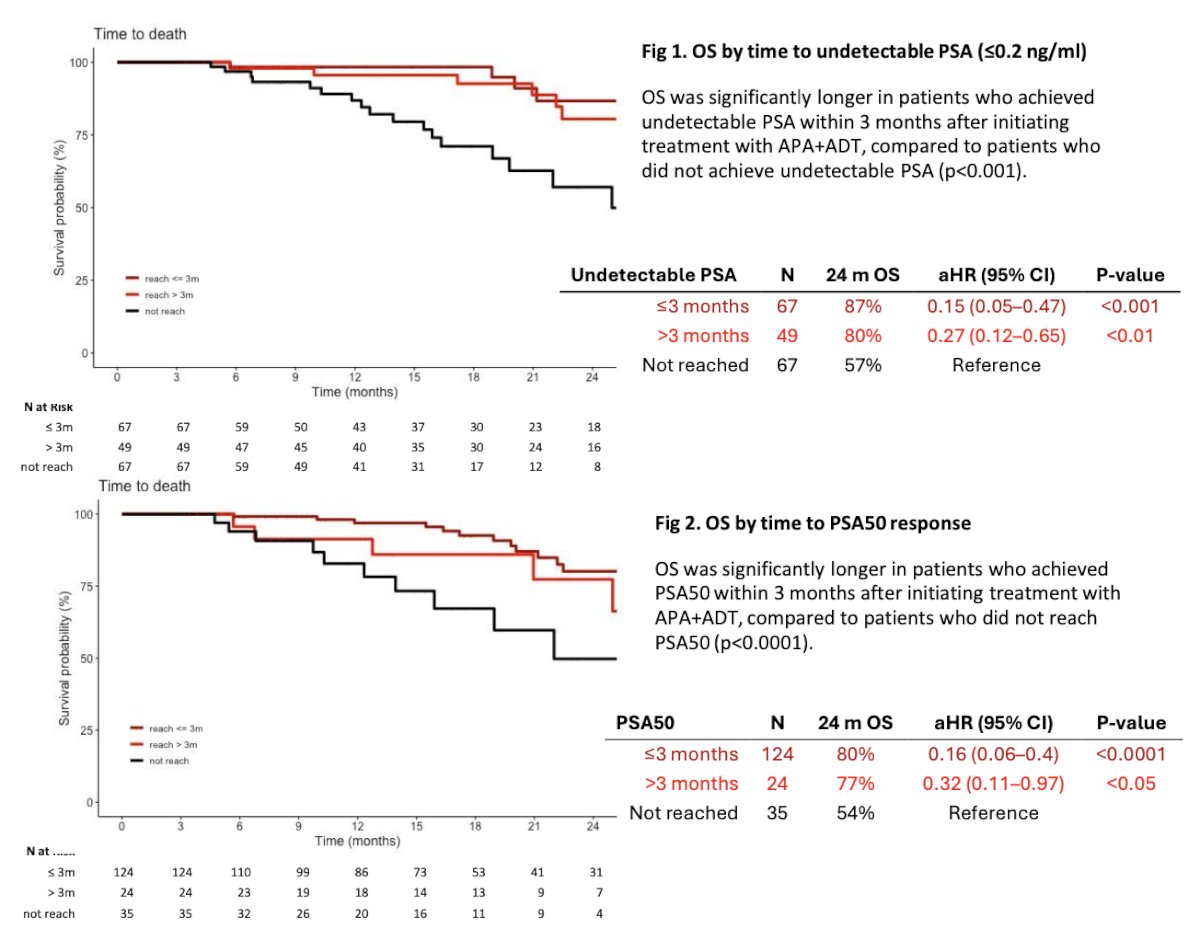
Dr. Maughan concluded his presentation with the following key takeaway points:
- Rapid and deep PSA responses with the use of Apalutamide+ADT as the starting therapy for mCSPC was associated with significantly improved survival in real-world practice (OASIS project)
- This real-world data confirms the favorable prognosis of PSA responses observed in prior prospective studies
- The speed and depth of the PSA response to Apalutamide+ADT positively impacts survival in patients with mCSPC.
Presented by: Benjamin L. Maughan, MD, PharmD, Assistant Professor, Genitourinary Medical Oncologist, Huntsman Cancer Institute, The University of Utah, Salt Lake City, UT
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31st and June 4th.
Related content: OASIS Project Highlights the Survival Benefits of Rapid PSA Decline with Apalutamide in Prostate Cancer - Benjamin Maughan
References:
- Chi KN, Chowdhury S, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, Given R, Juárez A, Merseburger AS, Özgüroğlu M, Uemura H, Ye D, Brookman-May S, Mundle SD, McCarthy SA, Larsen JS, Sun W, Bevans KB, Zhang K, Bandyopadhyay N, Agarwal N. Apalutamide in Patients With Metastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study. J Clin Oncol. 2021 Jul 10;39(20):2294-2303. doi: 10.1200/JCO.20.03488. Epub 2021 Apr 29. PMID: 33914595.
- Chowdhury et al. Deep, rapid, and durable prostate-specific antigen decline with apalutamide plus androgen deprivation therapy is associated with longer survival and improved clinical outcomes in TITAN patients with metastatic castration-sensitive prostate
- Facchini et al. Very early PSA Response to abiraterone in mCRPC patients: A novel prognostic factor predicting overall survival. Front Pharmacol. 2016; 18;7:123


