(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31 and June 4 was host to the Poster Session: Genitourinary Cancer: Prostate, Testicular, and Penile. Dr. Edmond Kwan presented an exploratory analysis assessing Circulating tumour DNA fraction (%) as a predictor of treatment efficacy in a randomized phase 2 trial of [177Lu] Lu-PSMA-617 (LuPSMA) versus cabazitaxel in metastatic castration-resistant prostate cancer (mCRPC) progressing after docetaxel (TheraP ANZUP 1603 trial).
The TheraP ANZUP 1603 trial (NCT03392428) was the first randomized trial to assess theranostic treatment using 177Lu-PSMA-617 (LuPSMA), a beta-emitter lutetium targeted to prostate cancer cells using antibodies to the transmembrane protein PSMA (prostate-specific membrane antigen), in men with metastatic castration-resistant prostate cancer (mCRPC).1 TheraP showed that in men with mCRPC who had disease progression after docetaxel, those randomly assigned to LuPSMA had significant improvements in PSA response rate (66% vs. 37%), RECIST response rate (49% vs. 24%), and progression-free survival (HR 0.63) compared to those who received cabazitaxel.2 The trial design is illustrated below: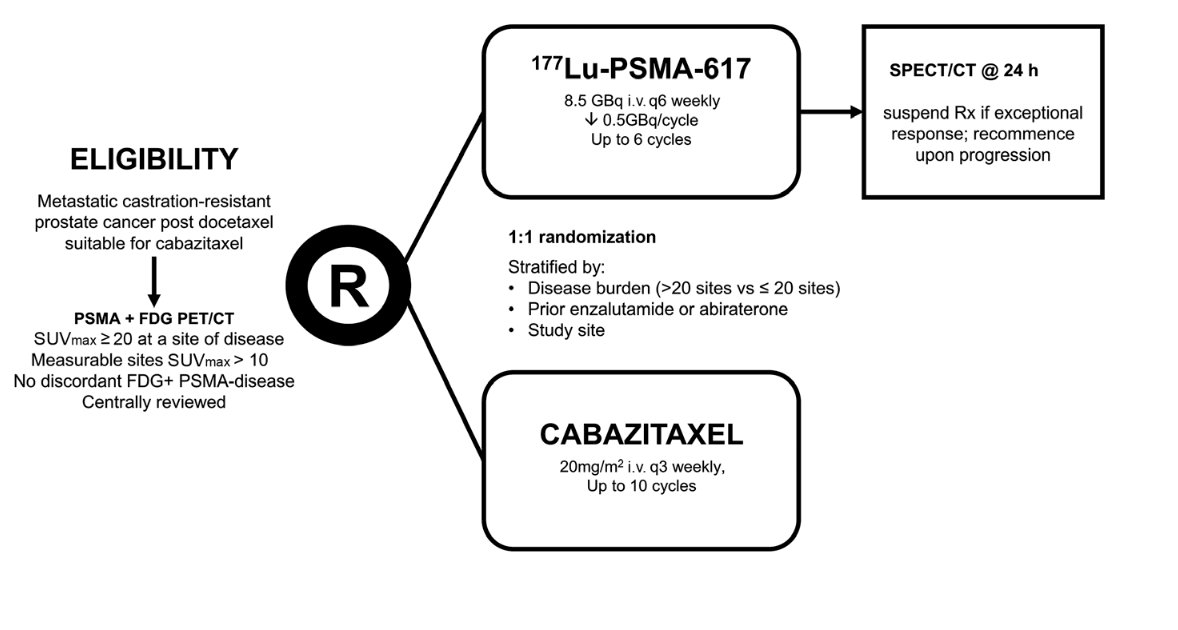
At this year's ASCO meeting, Dr. Kwan presented results concerning an exploratory analysis of TheraP, aiming to address the unmet clinical need of utilizing genomic data to guide optimal selection between LuPSMA and Cabazitaxel in patients with mCRPC. They explored the role of ctDNA fraction (ctDNA%) in baseline samples from the TheraP trial.
This exploratory analysis included 180 baseline blood samples from participants who received ≥1 cycle of protocol-assigned treatment. Plasma cell-free DNA and white blood cell DNA underwent targeted sequencing to estimate ctDNA% using a prostate cancer-focused panel.3 Prespecified ctDNA% categories (<2%, 2-30%, and >30%) were associated with previously validated imaging thresholds (PSMA SUVmean ≥10 and FDG metabolic tumor volume [MTV] ≥200mL),4 rate of PSA reduction ≥50%, and progression-free survival (PFS). Blood was extracted per protocol at baseline (pre-treatment), on-treatment at week 12 and after progression (end of treatment). The study design of this exploratory analysis is demonstrated in the following figure:
This analysis included 97 patients in the LuPSMA arm and 83 in the Cabazitaxel arm. Patient characteristics: age, disease burden, PSA, disease extent, and prior ARPI therapy appeared well balanced. The FDG PET MTV and proportion of patients with a PSMA SUVmean ≥10 was higher in the LuPSMA arm. Patient characteristics are illustrated in Table 1 below.
ctDNA% was evaluable in 178 (99%) participants. They found ctDNA ≥2% in 84% of participants, and the median ctDNA was 24%.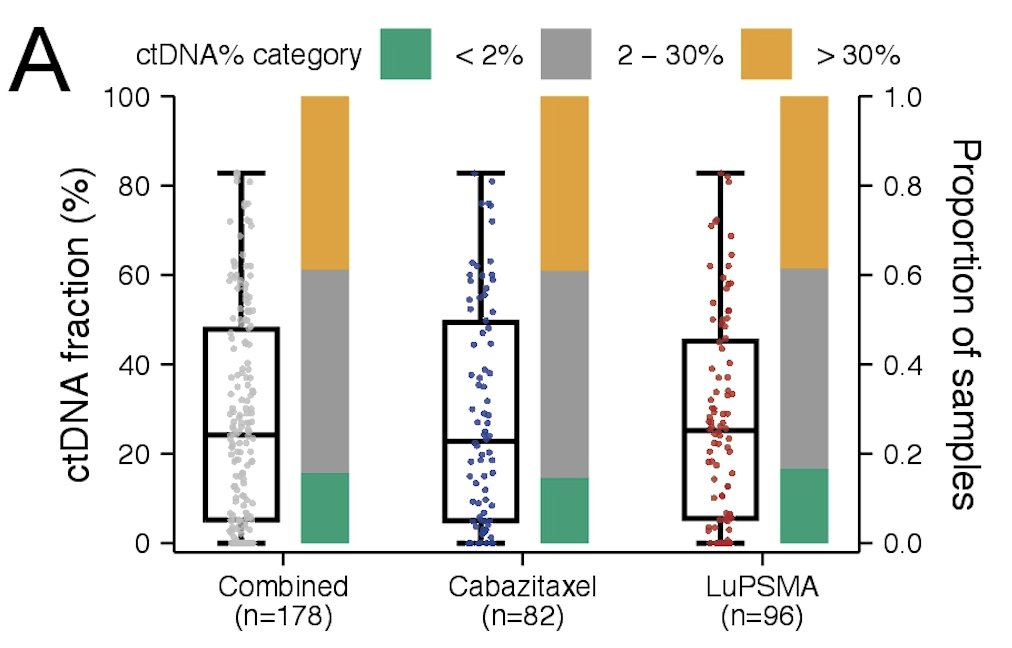
Higher ctDNA% categories (2-30%, >30%) were enriched for patients with high FDG MTV and low PSMA SUVmean disease.
The odds of a PSA50 (PSA decline of ≥50%) response to LuPSMA vs. cabazitaxel were significantly higher for men with ctDNA <2% (p=0.0067), with no significant difference at ctDNA >30% (OR 1.1, 95% CI 0.42-2.8). The PSA50 and PSA90 response stratified by ctDNA% categories are depicted below.
ctDNA <2% was associated with a significant improvement when treated with LuPSMA compared with Cabazitaxel, with an 8.7-month increase in the median PFS (HR 0.12, 95% CI 0.04-0.38, p=0.00025). This was confirmed on multivariable analysis adjusting for PSMA SUVmean and other clinical prognostic factors (HR 0.35, 95% CI 0.13-0.93; interaction p = 0.035). Kaplan-Meier graphics and forest plot of PFS in Cabazitaxel and LuPSMA arms stratified by ctDNA% groups are shown below.


The predictive potential of ctDNA% was additive to PSMA SUVmean in LuPSMA-treated patients.

Dr. Kwan wrapped up his presentation concluding:
- ctDNA% is associated with FDG MTV and low PSMA expression in mCRPC.
- ctDNA% could play a role as an adjunctive tool to aid patient selection for PSMA radioligand therapy.
- LuPSMA is associated with greater activity (PSA 50 response) with lower ctDNA%.
- ctDNA% is a candidate predictive and prognostic biomarker for differential response to LuPSMA vs. cabazitaxel in patients with molecular imaging-selected mCRPC progressing after.
- These findings are exploratory and require validation in independent cohorts
Presented by: Edmond Michael Kwan, PhD, MBBS, FRACP, Vancouver Prostate Centre, Department of Urologic Sciences, University of British Columbia, Canada.
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31st and June 4th.
Related content: ctDNA Fraction Predicts Response to Lutetium PSMA in TheraP Trial for mCRPC - Edmond Kwan
References:
- Hofman MS, Emmett L, Violet J, Y Zhang A, Lawrence NJ, Stockler M, Francis RJ, Iravani A, Williams S, Azad A, Martin A, McJannett M; ANZUP TheraP team; Davis ID. TheraP: a randomized phase 2 trial of 177Lu-PSMA-617 theranostic treatment vs cabazitaxel in progressive metastatic castration-resistant prostate cancer (Clinical Trial Protocol ANZUP 1603). BJU Int. 2019 Nov;124 Suppl 1:5-13. doi: 10.1111/bju.14876. Epub 2019 Oct 22. PMID: 31638341.
- Hofman MS, Emmett L, Sandhu S, Iravani A, Joshua AM, Goh JC, Pattison DA, Tan TH, Kirkwood ID, Ng S, Francis RJ, Gedye C, Rutherford NK, Weickhardt A, Scott AM, Lee ST, Kwan EM, Azad AA, Ramdave S, Redfern AD, Macdonald W, Guminski A, Hsiao E, Chua W, Lin P, Zhang AY, McJannett MM, Stockler MR, Violet JA, Williams SG, Martin AJ, Davis ID; TheraP Trial Investigators and the Australian and New Zealand Urogenital and Prostate Cancer Trials Group. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021 Feb 27;397(10276):797-804. doi: 10.1016/S0140-6736(21)00237-3. Epub 2021 Feb 11. PMID: 33581798.
- Tolmeijer SH, Boerrigter E, Sumiyoshi T, Kwan EM, Ng SWS, Annala M, Donnellan G, Herberts C, Benoist GE, Hamberg P, Somford DM, van Oort IM, Schalken JA, Mehra N, van Erp NP, Wyatt AW. Early On-treatment Changes in Circulating Tumor DNA Fraction and Response to Enzalutamide or Abiraterone in Metastatic Castration-Resistant Prostate Cancer. Clin Cancer Res. 2023 Aug 1;29(15):2835-2844. doi: 10.1158/1078-0432.CCR-22-2998. PMID: 36996325.
- Buteau JP, Martin AJ, Emmett L, Iravani A, Sandhu S, Joshua AM, Francis RJ, Zhang AY, Scott AM, Lee ST, Azad AA, McJannett MM, Stockler MR, Williams SG, Davis ID, Hofman MS; TheraP Trial Investigators and the Australian and New Zealand Urogenital and Prostate Cancer Trials Group. PSMA and FDG-PET as predictive and prognostic biomarkers in patients given [177Lu]Lu-PSMA-617 versus cabazitaxel for metastatic castration-resistant prostate cancer (TheraP): a biomarker analysis from a randomised, open-label, phase 2 trial. Lancet Oncol. 2022 Nov;23(11):1389-1397. doi: 10.1016/S1470-2045(22)00605-2. Epub 2022 Oct 16. PMID: 36261050.


