(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured a session on prostate cancer trials in progress, and a presentation by Dr. Elisabeth I. Heath discussing the trial design of CELC-G-201, a phase 1/2, open-label, randomized, dose-finding and dose expansion study of gedatolisib in combination with darolutamide in metastatic castration-resistant prostate cancer (mCRPC). Progression to mCRPC occurs in most patients treated with androgen receptor signaling inhibitors for advanced disease. Preclinical studies demonstrate that the androgen receptor and PI3K-AKT-mTOR (PAM) pathways interact through reciprocal negative feedback, and androgen receptor signaling inhibitor resistance can be induced through activation of PAM signaling:
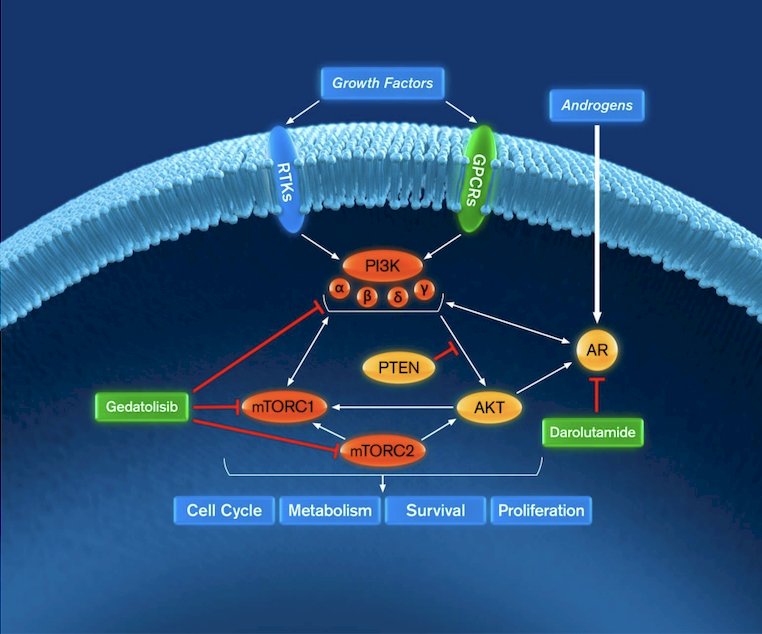
Thus, combining a PAM inhibitor with an androgen receptor signaling inhibitors may deliver improved anti-cancer activity in patients with mCRPC. A phase 2 trial in 129 patients with mCRPC who progressed on abiraterone demonstrated improved median radiographic progression-free survival when samotolisib, a dual PI3K-mTOR inhibitor, was added to enzalutamide. These results form the basis for the CELC-G-201 clinical trial of gedatolisib, a potent PAM inhibitor, in combination with darolutamide in men with mCRPC who have previously progressed on androgen receptor signaling inhibitors.
This open-label, multicenter, phase 1/2 study will evaluate the safety and efficacy of gedatolisib in combination with darolutamide in men with mCRPC who have progressed on androgen receptor signaling inhibitors. In phase 1, 36 patients will be randomized to one of two dose arms to evaluate dose limiting toxicities and determine the recommended phase 2 dose. Gedatolisib will be administered once weekly for 3-weeks-on/1-week-off: Arm 1 – 120 mg, and Arm 2 – 180 mg, with darolutamide 600 mg orally administered twice daily. Arm 2 may be dose de-escalated depending on the number of dose limiting toxicities observed. In phase 2, 12 additional patients will be enrolled at the recommended phase 2 dose (n = 30), for a total of approximately 48-54 patients. The trial design for CELC-G-201 is as follows:
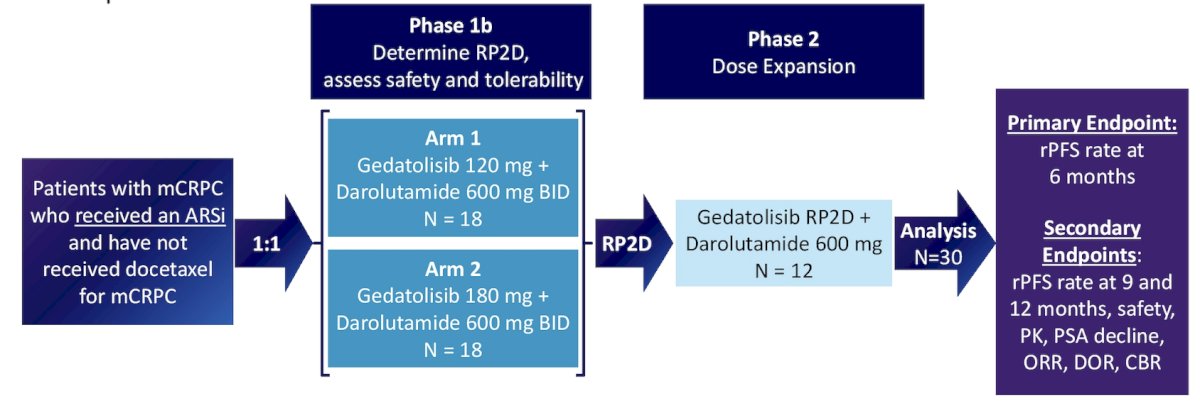
Key inclusion criteria include adult males (≥ 18 years) with mCRPC who have progressed on or after treatment with one next-generation androgen receptor signaling inhibitors. Key exclusion criteria include (i) males with adenocarcinoma with a small cell component and with ≥10% neuroendocrine type cells, (ii) prior treatment with PI3K, AKT, or mTOR inhibitor (iii) prior chemotherapy or radiopharmaceutical therapy for mCRPC (iv) uncontrolled type 1/2 diabetes (v) or active brain or leptomeningeal metastases.
Primary endpoints for phase 1 are safety and tolerability (incidence of dose limiting toxicities, adverse events, and determination of maximum tolerated dose) of gedatolisib + darolutamide for determination of the recommended phase 2 dose, pharmacokinetics, and Bayesian Optimal Interval utility score. Primary endpoint for phase 2 are radiographic progression-free survival rate at 6 months based on RECIST v1.1 with modifications as specified in PCWG3 criteria. Secondary endpoints include:
- Radiographic progression-free survival rates at 9 and 12 months
- Overall radiographic progression-free survival
- PSA response of ≥ 50% decrease from baseline at 4, 8, 12, and 16 weeks
- Overall response rate
- Duration of response
- Clinical benefit rate
- Overall survival rate at 18 and 24 months
- Safety
The CELC-G-201 trial is currently open for enrollment (NCT06190899) in the United States and Europe.
Presented by: Elisabeth I. Heath, MD, FACP, Barbara Ann Karmanos Cancer Institute, Wayne State University School of Medicine, Detroit, MI
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.


