(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured a session on prostate cancer, and a presentation by Dr. Marc-Oliver Grimm discussing a post hoc analysis of ARASENS discussing post-progression survival of patients with metastatic hormone-sensitive prostate cancer (mHSPC) who received darolutamide or placebo. Darolutamide is a structurally distinct and highly potent androgen receptor pathway inhibitor with low blood brain barrier penetration and limited potential for drug-drug interactions. In ARASENS, the addition of darolutamide to ADT and docetaxel significantly reduced the risk of death by 32.5% in patients with mHSPC,1 despite most placebo patients (75.6%) receiving subsequent therapy. Darolutamide also delayed time to progression to metastatic castration-resistant prostate cancer (mCRPC; median, not reached vs 19.1 months for placebo), resulting in a longer time in mHSPC, which is associated with improved quality of life versus mCRPC. At ASCO 2024, Dr. Grimm and colleagues reported post-progression subsequent anticancer therapies and related survival from ARASENS.
Patients with mHSPC were randomized 1:1 to darolutamide 600 mg twice daily or placebo in addition to ADT + docetaxel. After treatment discontinuation, patients entered active and long-term survival follow-up periods during which assessments included subsequent therapies and survival outcomes. Post-progression survival was defined as time from first subsequent therapy to death using Kaplan-Meier estimates.
Among 1,305 treated patients (darolutamide n = 651; placebo n = 654), 315 receiving darolutamide and 495 receiving placebo entered follow-up. Among these patients, 57% (n = 179) and 76% (n = 374), respectively, received subsequent therapy. This included abiraterone and enzalutamide as the most frequent first subsequent therapy:
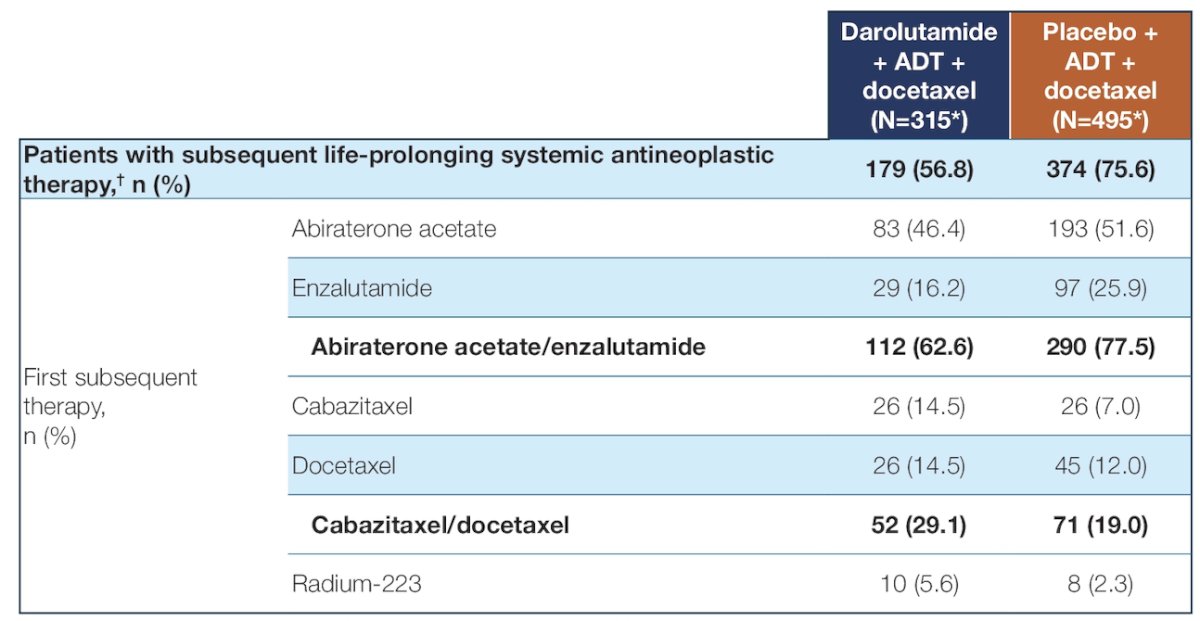
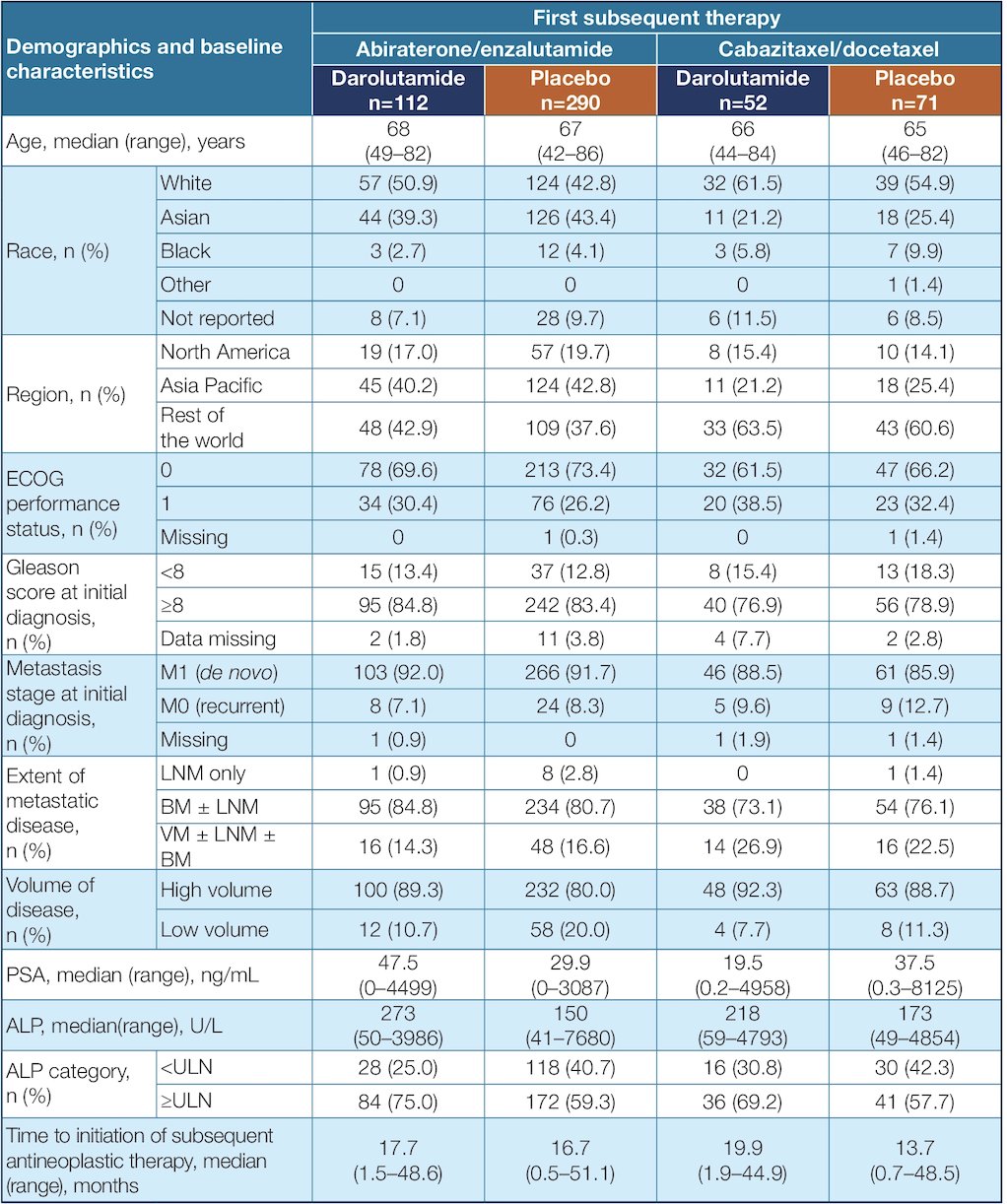
The five most common post-progression first subsequent anticancer therapies (in descending order) were abiraterone, enzalutamide, cabazitaxel, docetaxel, and radium-223. In the darolutamide arm, 90% of first subsequent therapies were androgen receptor pathway inhibitor or chemotherapy. In contrast, in the placebo arm, the majority (78%) received first subsequent therapy with either enzalutamide or abiraterone. Patient demographics and baseline characteristics were generally similar between the darolutamide and placebo groups within the first subsequent therapy subpopulations:
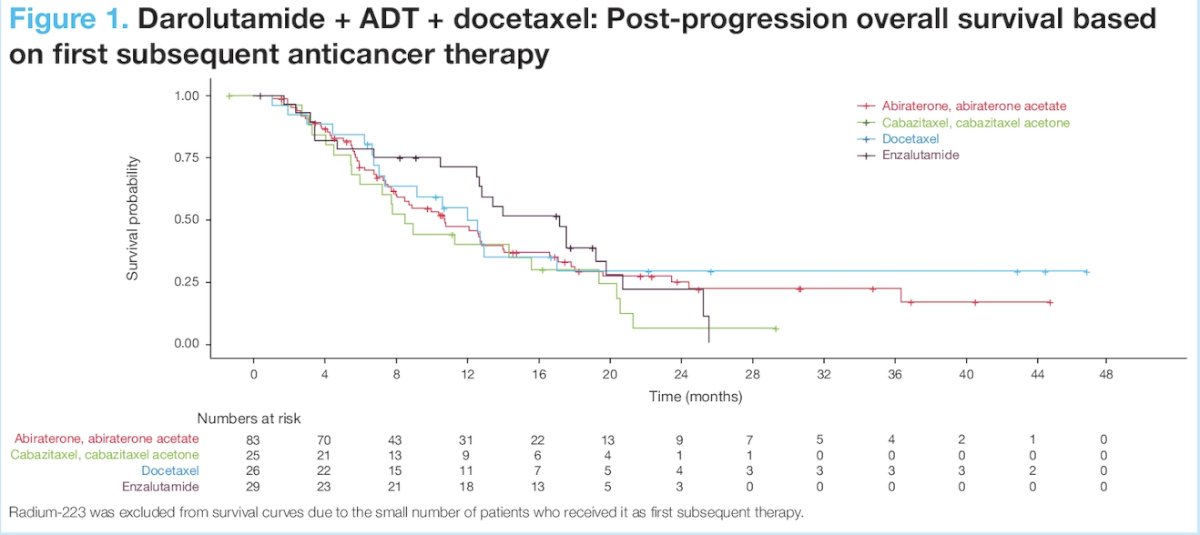
For patients receiving darolutamide + ADT + docetaxel, minimal difference was observed in post-progression survival between subsequent therapies, suggesting subsequent therapy with another androgen receptor pathway inhibitor does not provide further survival benefit vs non-androgen receptor pathway inhibitor options (mainly chemotherapy):
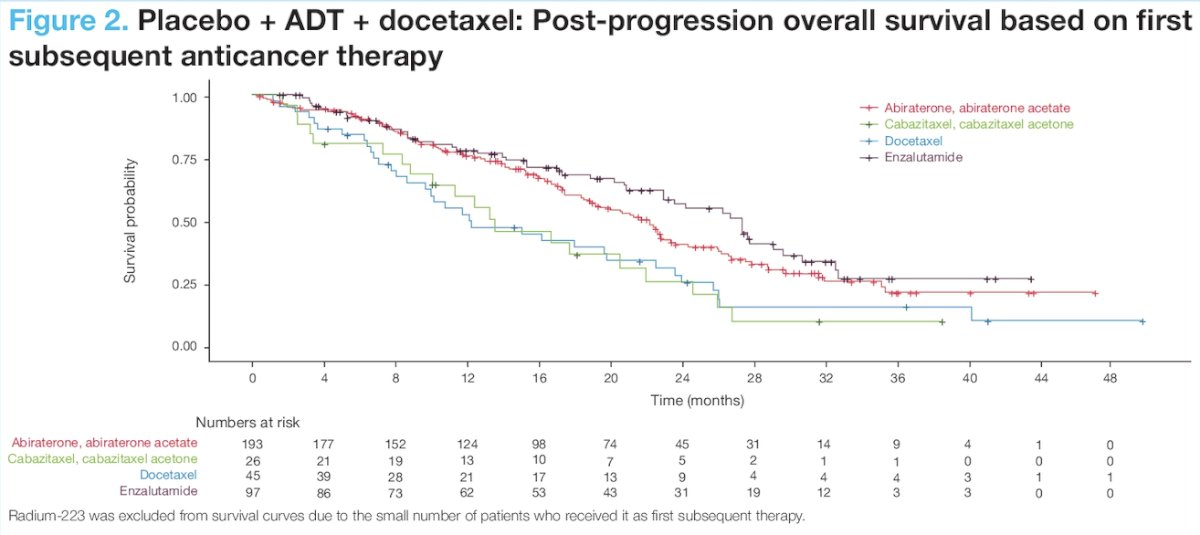
For patients who received placebo + ADT + docetaxel, post-progression survival was improved when the majority (78%) received a first subsequent anticancer therapy with a different mechanism of action. The median post-progression survival was 23.0 months with androgen receptor pathway inhibitor therapy and 13.5 months with non-androgen receptor pathway inhibitor subsequent therapy:
Patients receiving darolutamide + ADT + docetaxel stayed on treatment for more than 2 years longer than those receiving placebo + ADT + docetaxel, and median overall survival time was not reached in the darolutamide group:
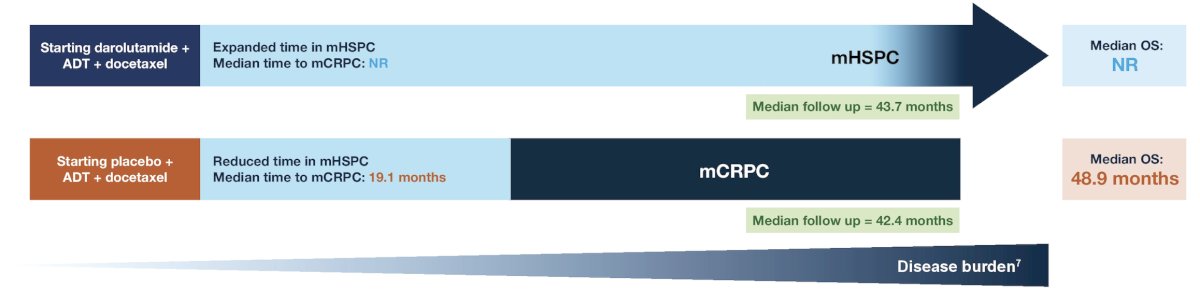
Dr. Grimm concluded his presentation by discussing a post hoc analysis of ARASENS discussing post-progression survival of patients with mHSPC who received darolutamide or placebo with the following take home messages:
- Darolutamide + ADT + docetaxel increased overall survival versus placebo + ADT + docetaxel and also delayed time to progression to mCRPC
- For patients receiving darolutamide + ADT + docetaxel for mHSPC, post-progression survival was similar independent of subsequent anticancer therapy with an androgen receptor pathway inhibitor or chemotherapy
- Patients receiving placebo + ADT + docetaxel mostly sequenced to an androgen receptor pathway inhibitor, and this first androgen receptor pathway inhibitor exposure provided a post-progression survival benefit versus subsequent non- androgen receptor pathway inhibitor therapies
- The greater overall survival benefit and delay in disease progression to mCRPC were observed when treatment was intensified with the darolutamide combination
Presented by: Marc-Oliver Grimm, MD, Jena University Hospital, Jena, Germany
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
References:


