(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured a session on prostate cancer trials in progress, and a presentation by Dr. Kim Chi discussing the trial design of EvoPAR-Prostate01, a phase 3, double-blind, placebo-controlled, 2-cohort, randomized study of saruparib (AZD5305) in combination with new hormonal agents in patients with metastatic castration-sensitive prostate cancer (mCSPC) with and without homologous recombination repair mutation. ADT + new hormonal agents have improved outcomes for patients with mCSPC, but patients will eventually progress to mCRPC, which is associated with poor survival. PARP inhibitors in combination with new hormonal agents are approved for treatment of metastatic castration-resistant prostate cancer (mCRPC). However, PARP inhibitor utilization in earlier lines of treatment may result in greater magnitude of benefit. Saruparib is a potential best-in-class PARP inhibitor, which selectively inhibits and traps PARP1, has minimal effect on PARP2, and hence may offer an improved therapeutic window compared with currently approved nonselective PARP inhibitors.
In the PETRA study, the favorable safety profile and low dose reduction rate observed with saruparib monotherapy compared with approved PARP inhibitors suggest that patients may be able to remain on treatment longer at an optimal dose (60 mg daily), which may improve efficacy. The efficacy and safety of saruparib + new hormonal agents for the treatment of mCSPC and mCRPC are being assessed in the phase I/IIa PETRANHA study (NCT05367440). Initial data from this study indicated that saruparib can be safely combined with enzalutamide, abiraterone acetate + prednisone or darolutamide. The phase III EvoPAR-Prostate01 study evaluates the efficacy and safety of saruparib + physician’s choice of new hormonal agents (abiraterone, darolutamide, or enzalutamide) compared with placebo + physician’s choice of new hormonal agents in participants with mCSPC.
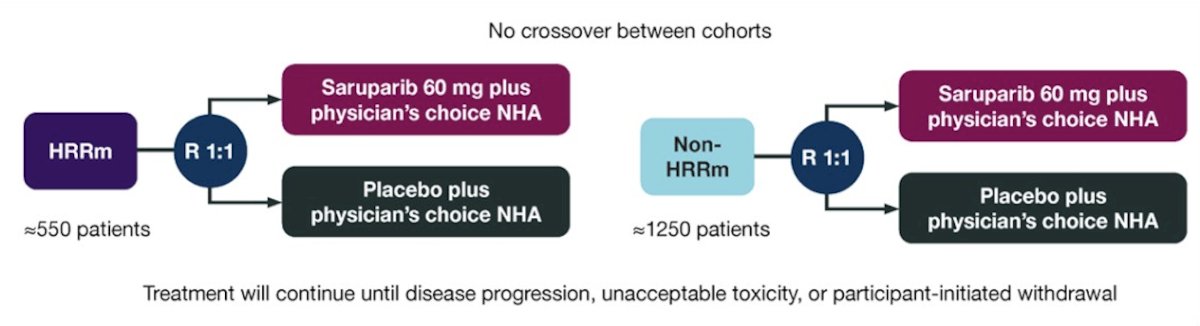
This is a 2-cohort, 2-arm, randomized, double-blind, placebo-controlled, multicenter global study with the following trial design:
Key eligibility criteria include age ≥18 years, histologically confirmed mCSPC (de novo or recurrent low- or high-volume disease), ECOG performance status 0-1, and confirmed, prospectively defined homologous recombination repair gene mutation status (defined by the presence/absence of pathogenic/likely pathogenic mutations in ≥1 of the genes BRCA1, BRCA2, ATM, CDK12, PALB2, RAD51B, RAD51C, RAD51D,and BARD1). Participants must be receiving ADT throughout the study or have undergone bilateral orchiectomy, and must be suitable for treatment with new hormonal agents. Key exclusion criteria include prior therapy with PARP inhibitors, prior chemotherapy or new hormonal agents in the mCSPC setting (prior new hormonal agents for localized disease permitted), and history of, or suspected, myelodysplastic syndrome/acute myeloid leukemia.
Participants are allocated to either the homologous recombination repair gene mutation or non-homologous recombination repair gene mutation cohort based on prospective testing of both tumor tissue and circulating tumor DNA. Participants are randomized 1:1 to receive saruparib + physician’s choice of new hormonal agent or placebo + physician’s choice of new hormonal agent. Treatment continues until disease progression, unacceptable toxicity, or participant-initiated withdrawal. The primary endpoint is radiographic progression-free survival, with overall survival a key secondary endpoint. Additional endpoints include:
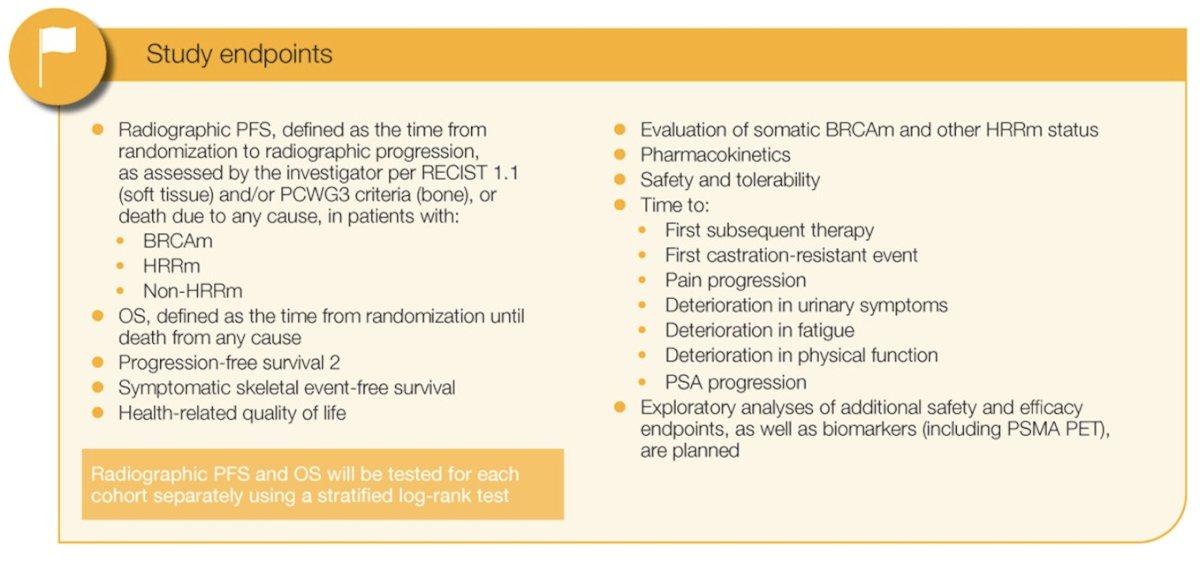
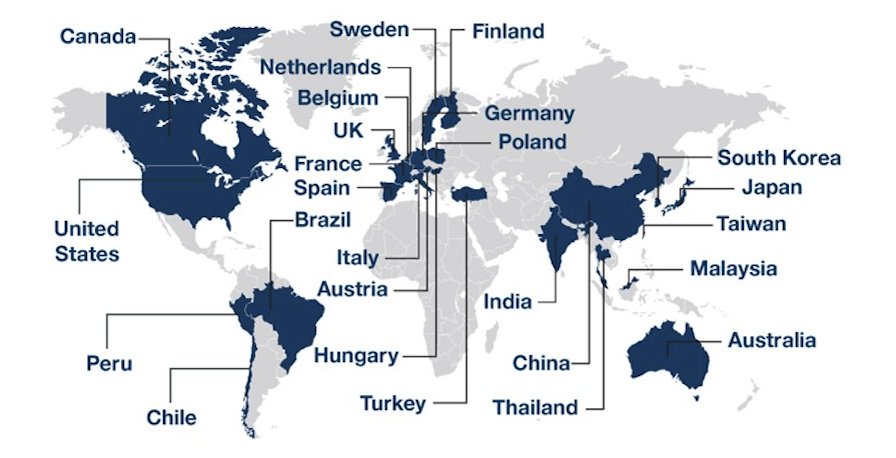
Planned statistical analyses of radiographic progression-free survival and overall survival will be conducted within each cohort using a stratified log-rank test. Approximately 1,800 participants (550 with homologous recombination repair gene mutations; 1,250 with non-homologous recombination repair gene mutation) will be randomized. Enrollment began in November 2023 and is ongoing. There are 370 study sites recruiting or planning to recruit patients from 26 countries across Asia-Pacific, Europe, North American, and South America:
Clinical trial information: NCT06120491.
Presented by: Kim N. Chi, MD, British Columbia Cancer Agency, Vancouver Cancer Centre, Vancouver, Canada
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.


