(Urotoday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31 and June 4 was host to the Poster Session: Genitourinary Cancer: Prostate, Testicular, and Penile. Dr. James Purtell presented the poster: Race and decisional conflict about genetic testing in patients with advanced prostate cancer.
Dr. Purtell commenced his presentation by highlighting that despite clinical practice guidelines recommending genetic testing in advanced prostate cancer, genetic testing remains underutilized and is not universally embraced by patients. For this research project, they evaluated patient attitudes and decisional conflicts regarding genetic testing, as well as the factors influencing subsequent test completion, including differences between white and non-white patients in the United States.
This prospective single-center study involved patients with stage IV prostate cancer who had not yet undergone genetic testing. Patients who chose to participate were administered a 24-question survey utilizing a Likert scale ranging from 0 (strongly agree) to 4 (strongly disagree) to gauge attitudes toward genetic testing. Additionally, a validated assessment of decisional conflict (DC) was conducted. DC and DC subscores, ranging from 0 to 100 (with 100 indicating the highest level of DC), were calculated from subsets of survey responses. The study design schema is illustrated below.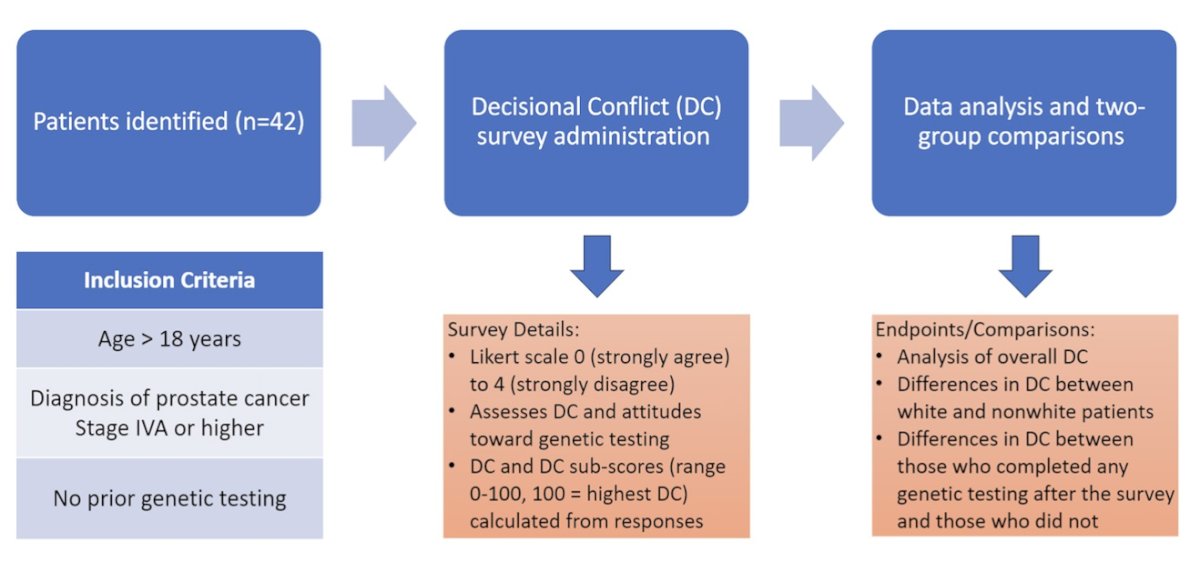
The patients' self-identified race was extracted from the electronic medical records. Two-group comparisons were conducted between white and non-white patients, as well as between those who completed genetic testing and those who did not.
Of the 42 enrolled patients, 21 were white, 17 black, 1 Asian, and 3 who declined to specify. Overall, 22 patients (52.4%) completed genetic testing. The survey questions and responses to the questions in percentages are shown in detail in the figure below.
Compared to white patients, non-white patients expressed greater concerns regarding test result privacy (mean = 1.72 vs. 2.95, p = 0.0015), potential non-healthcare uses of test results (1.78 vs. 3.00, p = 0.0028), and the possibility of trying unproven treatments (1.72 vs. 2.67, p = 0.0118). Additionally, non-white patients reported feeling more external pressure in decision-making compared to white patients (0.67 vs. 0.29, p = 0.037). However, no significant differences were observed in the completion of testing, decisional conflict (DC), or any DC subscore between racial groups.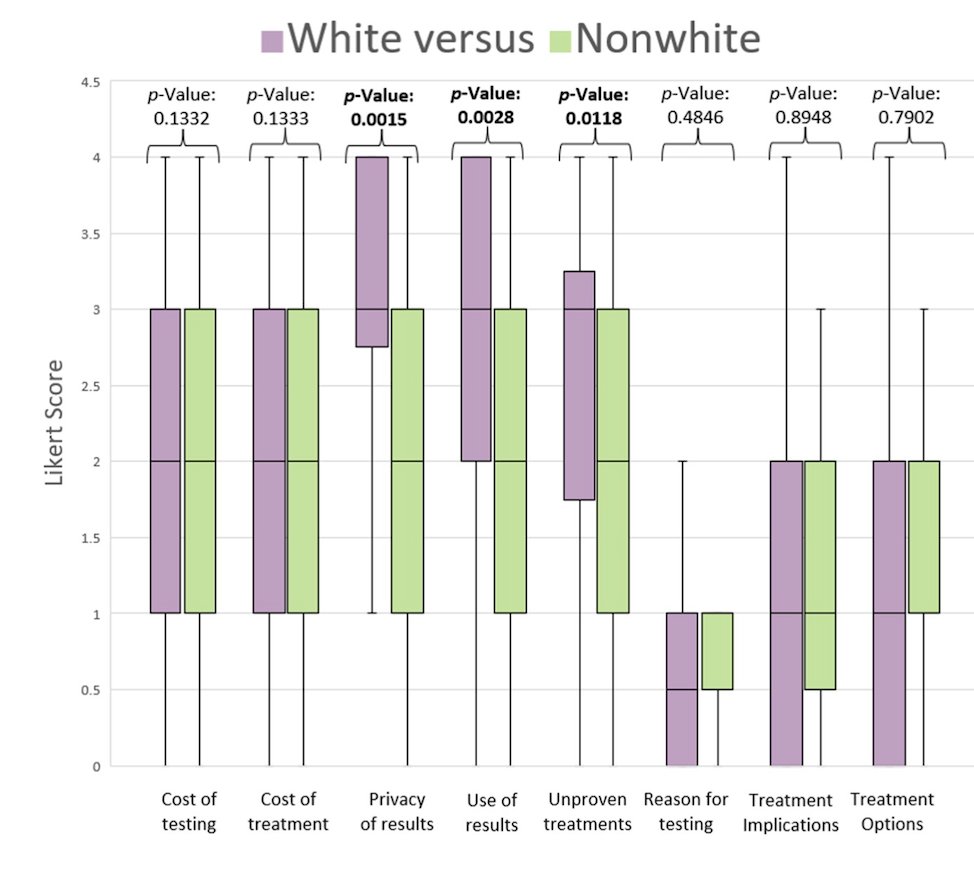
Compared to patients who did not complete testing, those who completed testing were:
- More likely to report they knew which options were available (0.73. v 1.25, p = 0.045)
- Knew the benefits of each option (0.77 v 1.30, p = 0.042)
- Knew the risk and side effects of each option (0.95 v 1.50, p = 0.046)
- Were clear about which benefits matter most to themselves (0.73 v 1.37, p = 0.016)
- Were clear about the best choice for themselves (0.73 v 1.35, p = 0.015).
Overall decisional conflict (28.59 v 18.11, p = 0.03) was higher in patients who did not complete testing, along with uncertainty (31.25 v 19.32, p = 0.02) and informed (31.25 v 20.45, p = 0.03) subscores.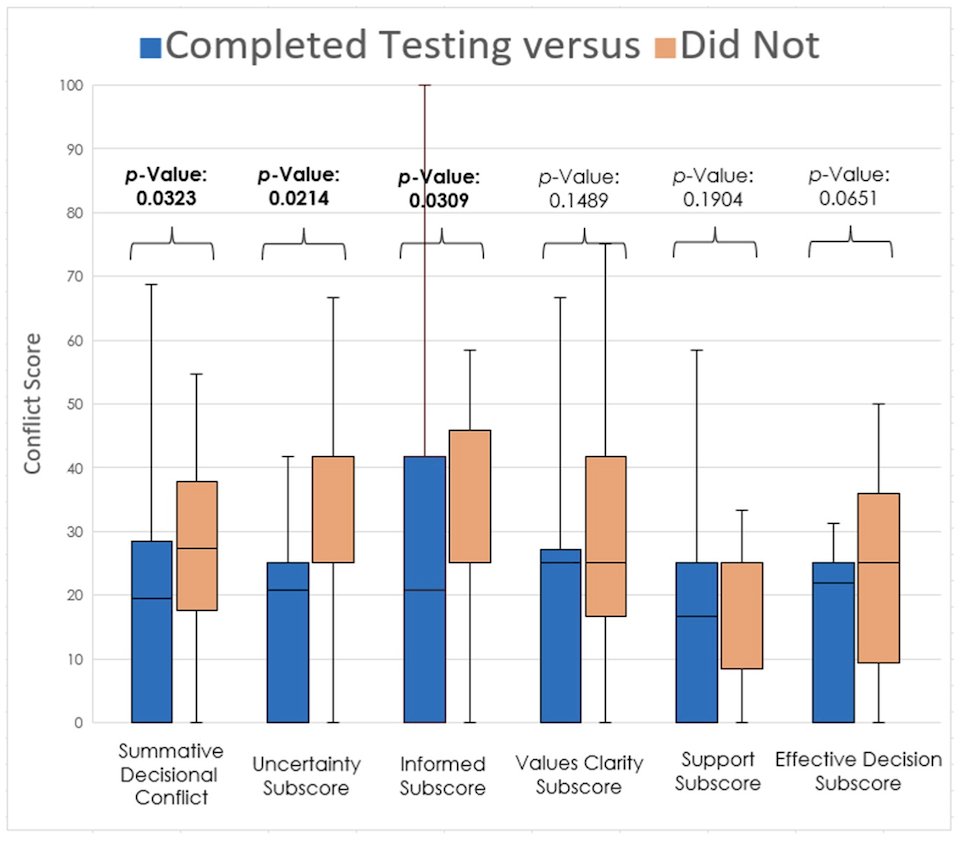
Dr. Purtell wrapped up their presentation concluding that according to their study:
- Non-white patients with stage IV prostate cancer expressed greater concern about privacy, data misuse, and trying unproven treatments.
- Those who did not complete testing had more DC with greater uncertainty about knowledge and decision-making.
- These findings will help direct targeted interventions to increase knowledge, trust, and decisional certainty about genetic testing in pts with advanced PC.
- Ongoing studies at Henry Ford Hospital will assess the impact of these interventions on rates of testing completion at our institution.
Presented by: James Purtell, MD, Internal Medicine Specialist, Henry Ford Hospital, Detroit, MI.
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31st and June 4th.


