(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31 and June 4 hosted the Poster Session: Genitourinary Cancer: Prostate, Testicular, and Penile, where Dr. Evan Yu presented the poster titled "Biomarkers associated with outcomes from KEYLYNK-010: Pembrolizumab (pembro) plus olaparib (ola) versus next-generation hormonal agent (NHA) in previously treated metastatic castration-resistant prostate cancer (mCRPC) patients.
Prostate cancer is characterized by significant heterogeneity, with various molecular pathways contributing to tumor progression and treatment resistance. The identification of biomarkers and actionable driver mutations capable of predicting responses to hormonal agents or systemic therapies remains an unmet need in the field.
The phase 3 open-label KEYLYNK-010 study (NCT03834519) evaluated pembrolizumab plus olaparib versus a next-generation hormonal agent (NHA) for biomarker-unselected, previously treated mCRPC. Eligible patients, aged ≥18 years, had mCRPC that progressed after abiraterone or enzalutamide (but not both), as well as docetaxel, and had an ECOG performance status ≤1. Patients were randomized 2:1 to receive either 200 mg pembrolizumab IV Q3W for ≤35 cycles + 300 mg olaparib orally BID, or a next-generation hormonal agent: 1000 mg abiraterone orally QD (if previously treated with enzalutamide) or 160 mg enzalutamide orally QD (if previously treated with abiraterone). The study design is depicted below: 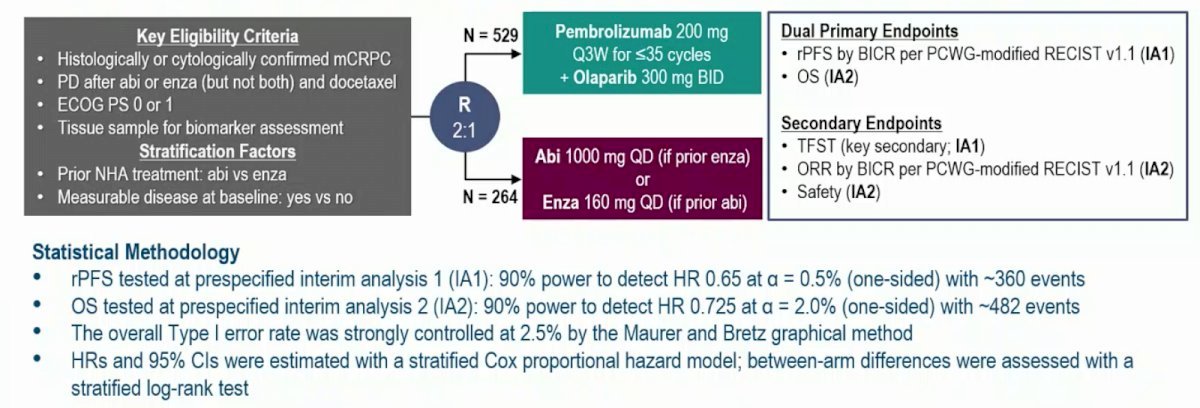
In the KEYLYNK-010 study, the combination of Pembrolizumab + olaparib did not show a significant improvement in rPFS or OS compared to NHA in unselected patients with previously treated mCRPC. The objective of this study was to assess the results of prespecified biomarker analyses from the KEYLYNK-010 study concerning rPFS or OS.
For this analysis, PD-L1 combined positive score (CPS) was measured by IHC (22C3 pharmDx). CPS was calculated as the number of PD-L1 staining cells (tumor cells, lymphocytes, macrophages) divided by the total number of tumor cells and multiplied by 100. Homologous recombination repair mutations (HRRm), BRCAm, TP53m, PTENm, SPOPm, ATMm, CDK12m were analyzed in tissue samples using FoundationOne CDx or ctDNA using FoundationOne Liquid CDx. Tumor mutational burden (TMB), microsatellite instability (MSI), and genomic loss of heterozygosity (gLOH) were analyzed in tumor tissue samples using FoundationOne CDx. Finally, AR-V7 was analyzed using Epic Sciences’ CTC assay.
The population for analysis were patients who received at least one dose of study treatment and had evaluable biomarker data. Subgroup analysis was performed for biomarkers with sufficient sample size and using the following pre-specified subgroups:
- PD-L1: CPS ≥1 versus <1
- HRR, BRCA, TP53, and PTEN: mutant versus wild type (WT)
- AR-V7 status: positive versus negative
- gLOH: ≥median score (7.8%) versus <median
The investigators explored the associations between biomarkers and survival outcomes using adjusted Cox proportional hazards regression. Specifically, for biomarkers with a sufficient sample size for subgroup analysis, they evaluated the hazard ratios (HRs) for rPFS and OS between pembrolizumab + olaparib and NHA.
In this exploratory analysis of the KEYLYNK-010 study, the investigators identified 782 treated patients. PD-L1 status was evaluable in 730 patients (490 receiving pembrolizumab + olaparib and 240 receiving NHA), while HRRm, BRCAm, TP53, PTEN, SPOP, ATM, and CDK12 status were evaluable in 718 patients (489 receiving pembrolizumab + olaparib and 229 receiving NHA). Other evaluable biomarkers according to the treatment arm are shown in the table below.
In the biomarker-evaluable population of the KEYLYNK-010 study, the prevalence of biomarkers and genomic features was:
- 24% PD-L1 CPS≥1
- 28% HRRm
- 10% BRCAm
- 45% TP53m
- 34% PTENm
- 8% SPOPm
- 7% ATMm
- 7% CDK12m
- 4% TMB-H (≥10 mut/Mb)
- 2% MSI-H
- 13% AR-V7 positive.
The distribution of biomarker and genomic features according to treatment arm is illustrated in the figure below: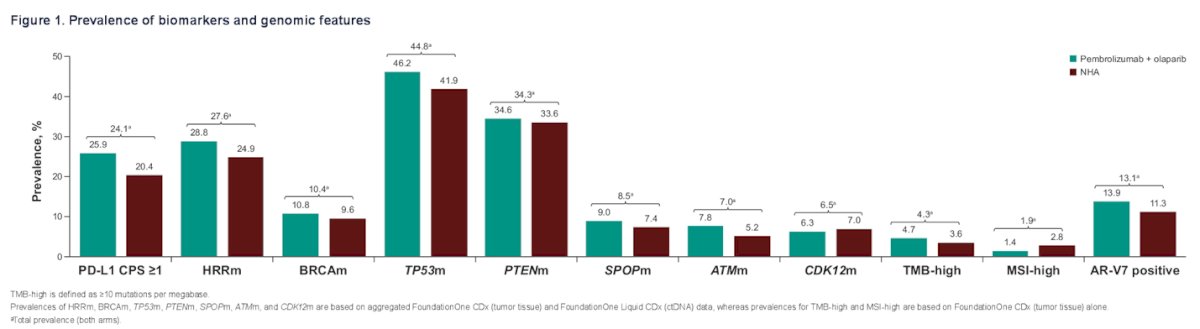
For Pembrolizumab + Olaparib, rPFS positive associations were only observed between PD-L1 (as a continuous variable) (p = 0.034), HRRm (p = 0.003), and BRCAm (p< 0.001, respectively). No associations were observed for any treatment arm/biomarker and overall survival (all p> 0.05).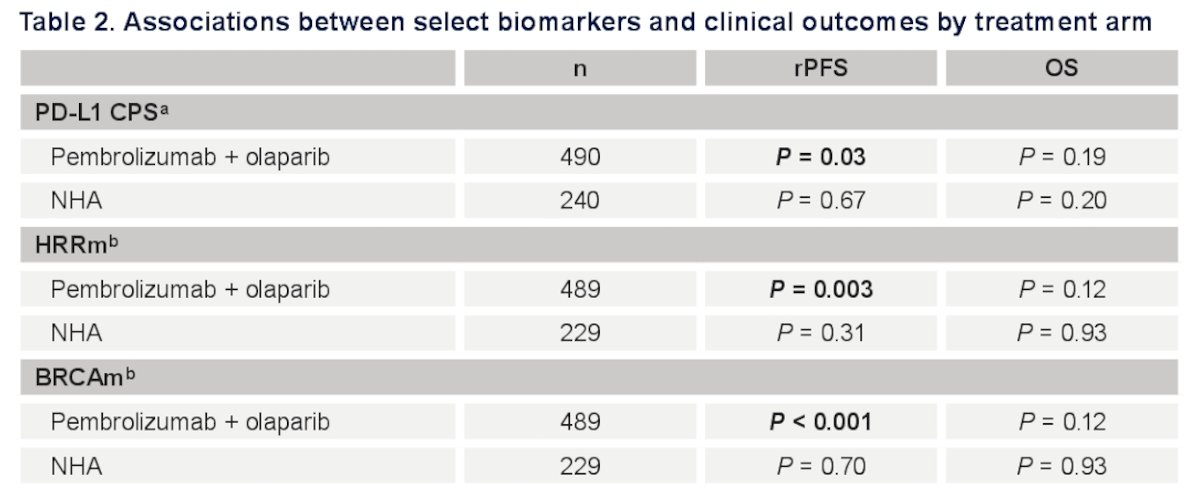
The hazard ratios and Kaplan-Meier curves for rPFS and OS, comparing pembrolizumab + olaparib with NHA across PD-L1 CPS≥1 and PD-L1 CPS<1 patients, are depicted below: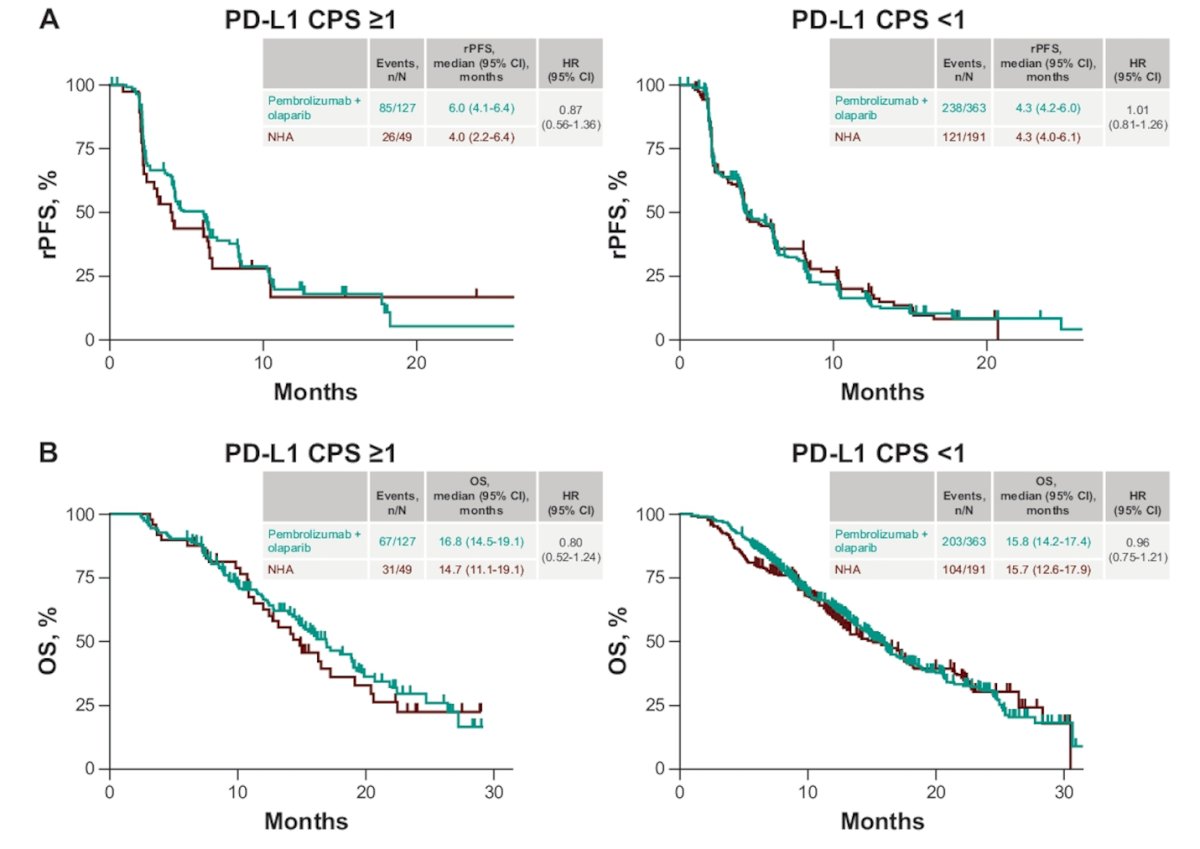
In BRCAm patients, Pembrolizumab + Olaparib was associated with significantly better rPFS (HR 0.43, 95% CI 0.23-0.83). However, the same benefit was not observed in OS.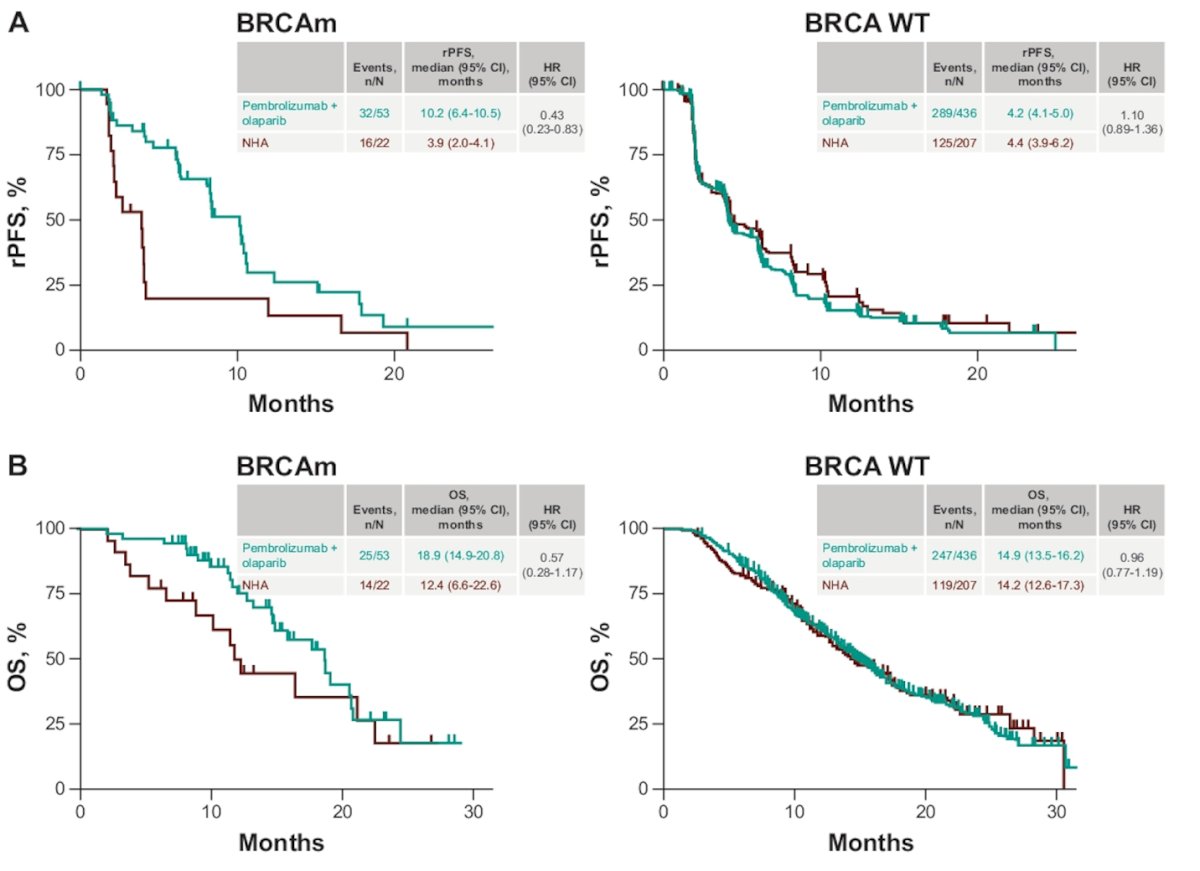
Notably, in the Pembrolizumab + Olaparib group, AR-V7 positivity exhibited a potential benefit in rPFS (HR 0.47, 95% CI 0.24-0.91) and OS (HR 0.41, 95% CI 0.22-0.78).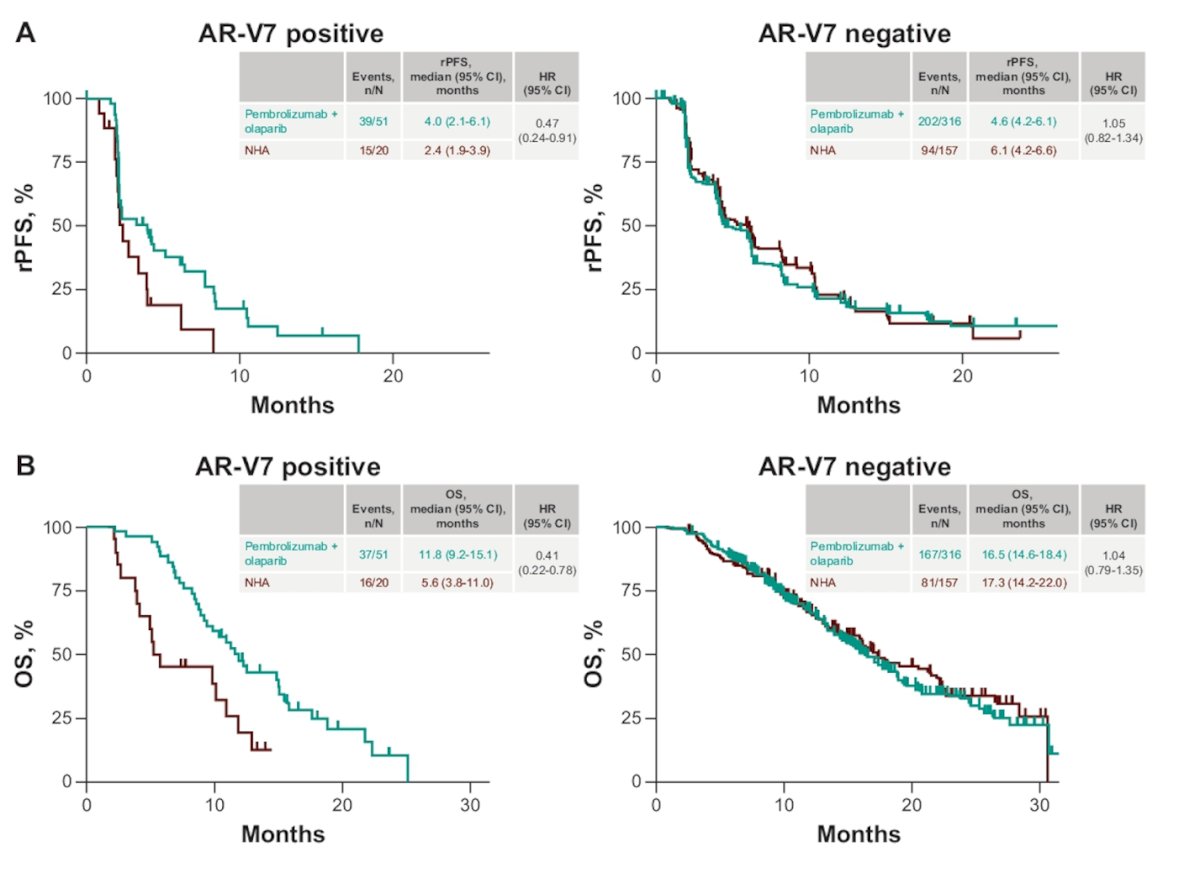
Dr. Yu concluded his presentation with the following messages:
- For pembrolizumab + olaparib, PD-L1 CPS (continuous variable), HRRm, and BRCAm showed positive associations with rPFS but no significant association with OS.
- Neither of these biomarkers correlated with NHA outcomes.
- A potential benefit with pembrolizumab + olaparib over NHA rPFS and OS) in AR-V7+ mCRPC patients was observed, but this requires further validation.
Presented by: Evan Y. Yu, MD, Medical Oncologist, Professor of Medicine, Division of Oncology, University of Washington School of Medicine, Section Head of Cancer Medicine, Clinical Research Division, Fred Hutchinson Cancer Center, The University of Washington School of Medicine, Seattle Cancer Care Alliance, Seattle, WA
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31st and June 4th.
Reference:


