(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31 and June 4 was host to the Poster Session: Genitourinary Cancer: Prostate, Testicular, and Penile. Dr. Ali Sabbagh developed a machine-learning model to predict overall survival endpoints from randomized clinical trials of patients with metastatic prostate cancer
Overall survival (OS) continues to serve as the gold standard endpoint for clinical trials in metastatic prostate cancer. However, due to the natural course of the disease and the emergence of innovative life-prolonging therapies, it requires prolonged follow-up. The objective of this project was to develop a machine learning model using short-term (≤ 4 months) prostate-specific antigen (PSA) kinetic data to forecast the OS outcome in phase 3 clinical trials for metastatic prostate cancer. The ultimate aim is to expedite the trial readout time for patients with metastatic prostate cancer.
This machine learning model utilized clinical and PSA data from 7 metastatic prostate cancer trials: 6 randomized, double-blind, phase 3 trials (TITAN, COU-AA-301, COU-AA-302, LATITUDE, ACIS, and MAGNITUDE),1-6 and one multicenter, phase II trial (GALAHAD).7 The trials were split into Training (TITAN, COU-AA-301, ACIS, GALAHAD) and test sets (LATITUDE, COU-AA-302, MAGNITUDE).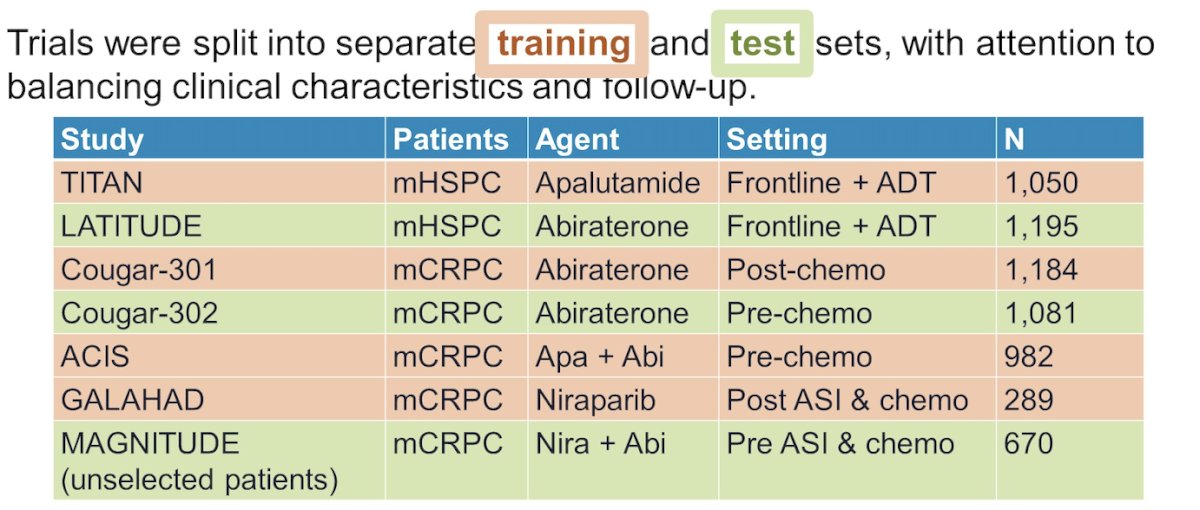
The OS probability of each trial is illustrated in the Kaplan-Meier graphic below: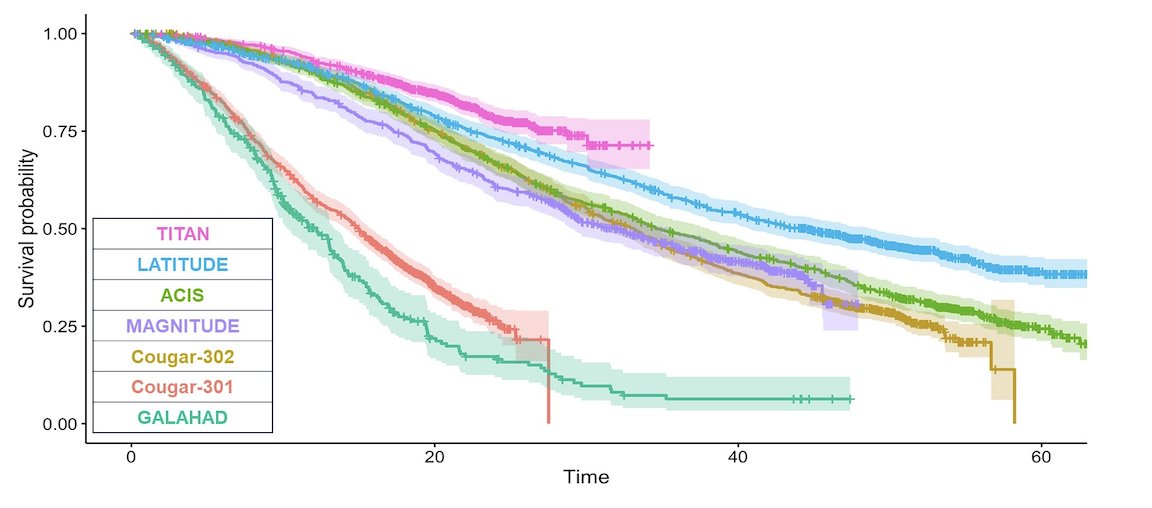
Dr. Sabbagh and his team developed 18 PSA kinetic variables from the first 4 months of enrollment, within 1, 2, 3, and 4 months; and the slope and base of the exponential fit of PSA over the first 4 months of study. The PSA kinetic variables were:
- 50% decline in PSA (PSA50)
- 90% decline in PSA
- PSA=0.1
- PSA=0.2
The model training was conducted on data from TITAN, COU-AA-301, ACIS, and GALAHAD trials, as mentioned above. Adaptive least absolute shrinkage and selection operator (aLASSO)-based Cox proportional hazards models were utilized to select previously identified prognostic baseline clinical variables, with and without PSA kinetic variables, that were most predictive of OS on five-fold cross-validation. The resulting trial outcome model was then evaluated for performance with different metrics (C-index, AUC) both with and without PSA kinetics. The simulated trial outcomes hazard ratios were pooled and compared with actual trial results using the methods of Crowther and Lambert.
External validation was carried out on data from LATITUDE, COU-AA-302, and MAGNITUDE trials. The algorithm was provided with 4-month PSA kinetics, baseline characteristics, and the trial outcome model. The resulting simulated trial outcomes were expressed as hazard ratios. The study design is illustrated below.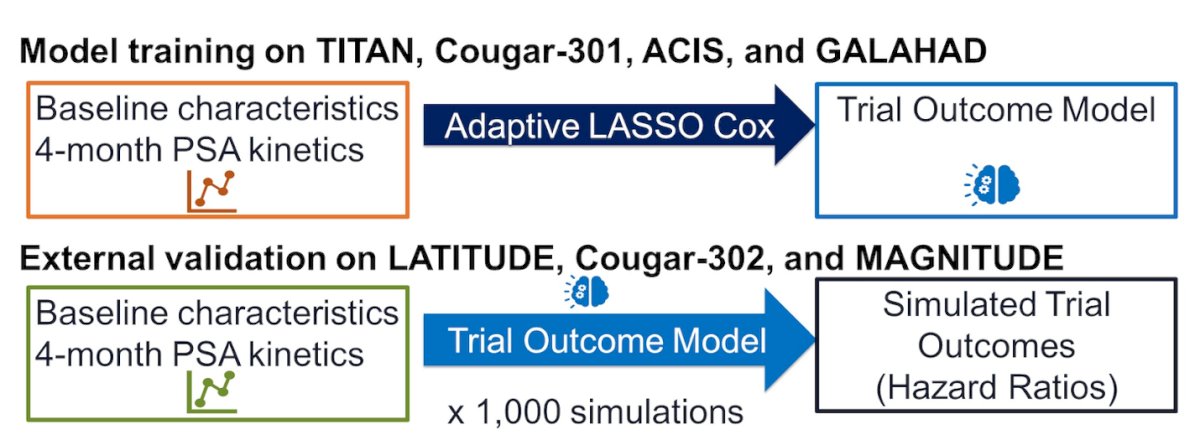
Including the 6 randomized clinical trials, this study included 6,451 patients with median follow-up of 22 months and a total of 85,795 PSA values. Dr Sabbagh noted that 4 months optimized the balance of predictive information and follow-up. Machine learning selected PSA kinetics based on three attributes:
- Absolute value
- Relative change
- Trajectory slope.
In the machine learning model incorporating PSA kinetics, aLASSO identified PSA50 at 4 months, PSA 0.1 at 1 month, and PSA slope as kinetics, providing additional intra-treatment information to enhance the prediction of OS. The model utilized PSA measurements at various intervals, as illustrated in the graphic below, depicting PSAs included for each patient in the external validation set.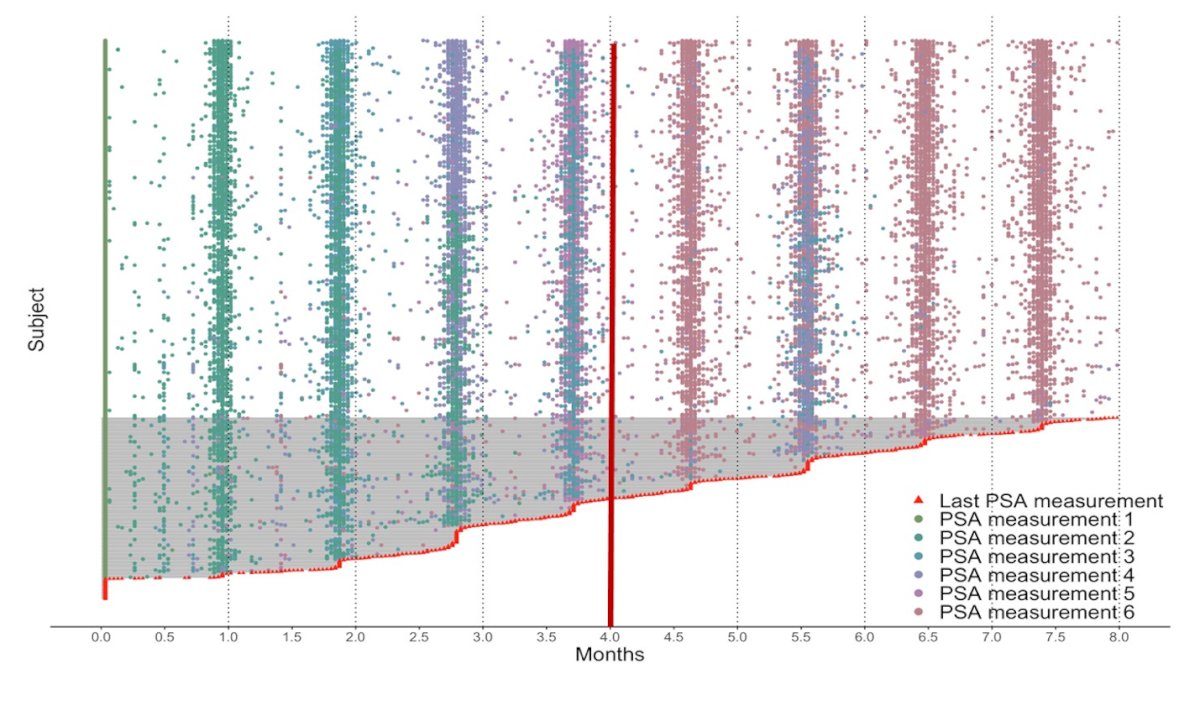
The machine learning model, for all three externally validated trials demonstrated accurate predictions of final positive or negative results. The hazard ratio estimates were distributed, showing significant overlap with the confidence intervals of the actual trial results. The model had almost perfect overlap with LATITUDE and different degrees of overlap with actual COU-AA-302 and MAGNITUDE hazard ratios and 95% confidence intervals as depicted in the figure below.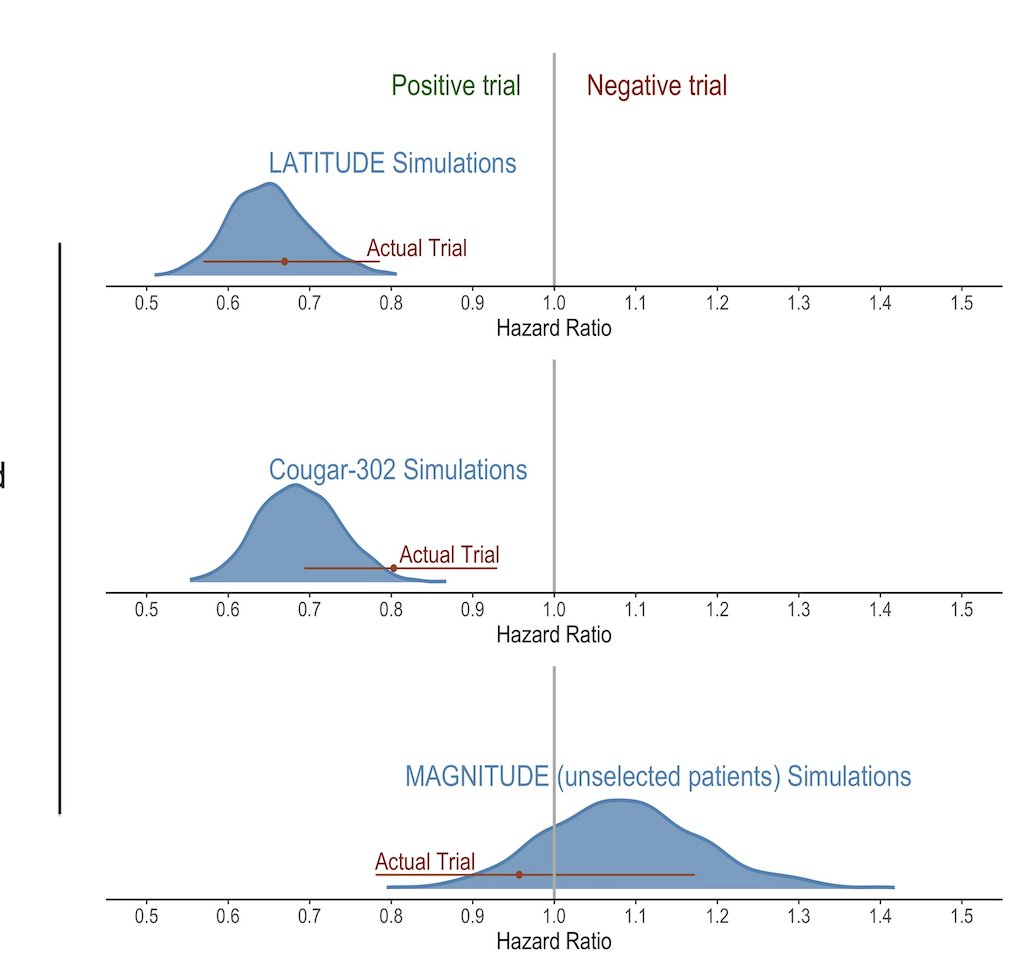
The model that included PSA kinetics showed improved performance on the externally validated trials:
- C-index (0.72 vs. 0.66)
- Integrated Brier score (IBS) (0.158 vs. 0.173)
- Time-dependent area under the receiver operating characteristic curve (tAUC) (0.84 vs 0.65 at 12 months)
One of the key strengths of this machine-learning model is that validation was demonstrated across a variety of clinical scenarios and patient populations including metastatic hormone sensitive and hormone-resistant prostate cancer.
Dr. Sabbagh concluded his presentation with the following key messages:
- Using data from 6,451 patients with metastatic prostate cancer enrolled on prospective clinical trials they develop a machine learning model which included 4-month kinetic PSA data and baseline characteristics
- The Machine learning model predicted the long-term OS readout in prospective phase 3 trials in metastatic prostate cancer across diverse clinical scenarios
- This model needs further validation and will be validated in independent data sets comprised of other clinical scenarios and subgroups
Presented by: Ali Sabbagh, MD, Postdoctoral Scholar, University of California, San Francisco, San Francisco, CA
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31st and June 4th.
References:
- Chi KN, Chowdhury S, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, Given R, Juárez A, Merseburger AS, Özgüroğlu M, Uemura H, Ye D, Brookman-May S, Mundle SD, McCarthy SA, Larsen JS, Sun W, Bevans KB, Zhang K, Bandyopadhyay N, Agarwal N. Apalutamide in Patients With Metastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study. J Clin Oncol. 2021 Jul 10;39(20):2294-2303. doi: 10.1200/JCO.20.03488. Epub 2021 Apr 29. PMID: 33914595.
- Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ, Staffurth JN, North S, Vogelzang NJ, Saad F, Mainwaring P, Harland S, Goodman OB Jr, Sternberg CN, Li JH, Kheoh T, Haqq CM, de Bono JS; COU-AA-301 Investigators. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012 Oct;13(10):983-92. doi: 10.1016/S1470-2045(12)70379-0. Epub 2012 Sep 18. Erratum in: Lancet Oncol. 2012 Nov;13(11):e464. Erratum in: Lancet Oncol. 2014 Aug;15(9):e365. PMID: 22995653.
- Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN, Miller K, Logothetis CJ, Shore ND, Small EJ, Carles J, Flaig TW, Taplin ME, Higano CS, de Souza P, de Bono JS, Griffin TW, De Porre P, Yu MK, Park YC, Li J, Kheoh T, Naini V, Molina A, Rathkopf DE; COU-AA-302 Investigators. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015 Feb;16(2):152-60. doi: 10.1016/S1470-2045(14)71205-7. Epub 2015 Jan 16. PMID: 25601341.
- Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, Özgüroğlu M, Ye D, Feyerabend S, Protheroe A, De Porre P, Kheoh T, Park YC, Todd MB, Chi KN; LATITUDE Investigators. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med. 2017 Jul 27;377(4):352-360. doi: 10.1056/NEJMoa1704174. Epub 2017 Jun 4. PMID: 28578607.
- Saad F, Efstathiou E, Attard G, Flaig TW, Franke F, Goodman OB Jr, Oudard S, Steuber T, Suzuki H, Wu D, Yeruva K, De Porre P, Brookman-May S, Li S, Li J, Thomas S, Bevans KB, Mundle SD, McCarthy SA, Rathkopf DE; ACIS Investigators. Apalutamide plus abiraterone acetate and prednisone versus placebo plus abiraterone and prednisone in metastatic, castration-resistant prostate cancer (ACIS): a randomised, placebo-controlled, double-blind, multinational, phase 3 study. Lancet Oncol. 2021 Nov;22(11):1541-1559. doi: 10.1016/S1470-2045(21)00402-2. Epub 2021 Sep 30. PMID: 34600602; PMCID: PMC9377412.
- Chi KN, Rathkopf D, Smith MR, Efstathiou E, Attard G, Olmos D, Lee JY, Small EJ, Pereira de Santana Gomes AJ, Roubaud G, Saad M, Zurawski B, Sakalo V, Mason GE, Francis P, Wang G, Wu D, Diorio B, Lopez-Gitlitz A, Sandhu S; MAGNITUDE Principal Investigators. Niraparib and Abiraterone Acetate for Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol. 2023 Jun 20;41(18):3339-3351. doi: 10.1200/JCO.22.01649. Epub 2023 Mar 23. PMID: 36952634; PMCID: PMC10431499.
- Smith MR, Scher HI, Sandhu S, Efstathiou E, Lara PN Jr, Yu EY, George DJ, Chi KN, Saad F, Ståhl O, Olmos D, Danila DC, Mason GE, Espina BM, Zhao X, Urtishak KA, Francis P, Lopez-Gitlitz A, Fizazi K; GALAHAD investigators. Niraparib in patients with metastatic castration-resistant prostate cancer and DNA repair gene defects (GALAHAD): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2022 Mar;23(3):362-373. doi: 10.1016/S1470-2045(21)00757-9. Epub 2022 Feb 4. PMID: 35131040; PMCID: PMC9361481.


