(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31 and June 4 was host to the Poster Session: Genitourinary Cancer: Prostate, Testicular, and Penile. Dr. Wadih Issa presented the results of a retrospective study analyzing treatment outcomes for patients with Ga68-PSMA-PET prostate cancer with or without conventional imaging correlates.
Dr. Issa began his presentation by highlighting the significant burden of prostate cancer, ranking as the second-leading cause of cancer-related mortality. While conventional imaging modalities like CT and bone scans are routinely used for prostate cancer staging, they often lack sensitivity and specificity in detecting early metastatic disease, leading to a considerable rate of false-negative findings. Conversely, PSMA PET/CT has demonstrated high sensitivity and has transformed both treatment and staging approaches. However, it has also introduced a new paradigm, delineating a molecularly advanced disease space characterized by PSMA positivity and conventional imaging negativity (PSMA+/CT-), resulting in stage migration. Uncertainty persists regarding treatment outcomes and optimal management strategies for patients within this molecularly advanced disease space. Therefore, the objective of this study was to analyze treatment outcomes for patients undergoing Ga68-PSMA-PET imaging
This was a single-center retrospective analysis including patients with metastatic hormone-sensitive prostate cancer (mHSPC) who underwent PMSA PET imaging and received androgen deprivation therapy (ADT). PSMA PET at their institution used Ga68 gozetotide/PSMA-11. Patients were stratified into two groups by whether a CT lesion correlates to a PSMA avid lesion (SUVmax >2.5):
- PSMA+/CT-
- PSMA+/CT+
The definition for CT- was lymph nodes measuring less than 1.5 cm and exhibiting faint or undetermined sclerosis or bone lesions without a corresponding CT correlate. Conversely, CT+ was defined by lymph nodes larger than 1.5 cm and the presence of sclerotic lesions on CT or a positive bone scan. Patients were stratified based on the presentation of metastasis into de novo or metachronous categories and by the treatment received, including ADT alone, ADT combined with androgen receptor targeted therapies (ARTT), and ADT in combination with Docetaxel as illustrated in the figure below.
The primary objectives were PSA progression-free survival (PFS) and time to castration-resistant (TTCR) disease between the two groups. The secondary objective was the time to castration-resistant disease in patients with PSMA+/CT- disease, based on exposure to androgen deprivation therapy (ADT) alone or treatment intensification. The survival analysis was conducted using Kaplan-Meier and Cox proportional hazards regression to control for possible confounders.
A total of 159 patients were included in the analysis, of whom 104 received ADT, 44 (54%) in the PSMA+/CT- group compared to 64 (82%) in the PSMA+/CT+ group. Patients PSMA+/CT- group exhibited a lower median PSA level (1.1 vs. 15 ng/ml, p<0.001), a reduced incidence of de novo metastatic disease (37% vs. 59%, p=0.004), compared to the PSMA+/CT+ group.
PFS was significantly better for patients in the PSMA+/CT- group (p<0.01) compared to the PSMA+/CT+ group.
Similar the TTCR was significantly higher for patients in the PSMA+/CT- group (p<0.001) compared to the PSMA+/CT+ group.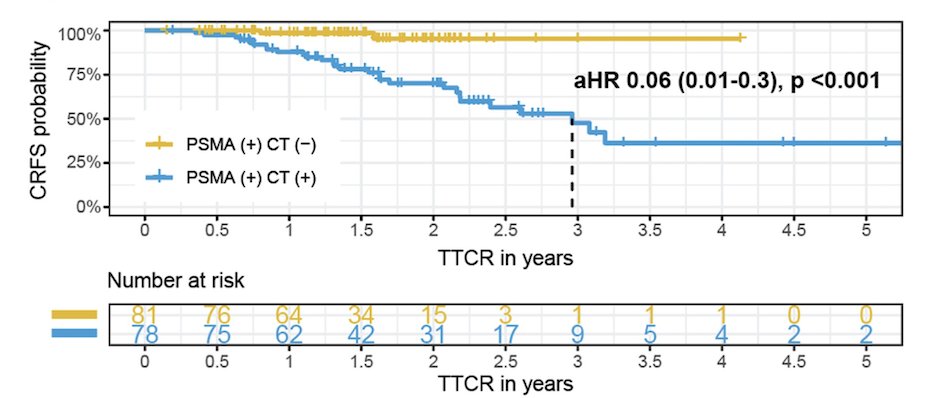
The TTCR disease was also explored based on treatment (ADT monotherapy vs. ADT intensification with ARTT or Docetaxel) in patients with PSMA+/CT-. Interestingly, the TTCR did not show significant differences regardless of treatment intensification in this retrospective study.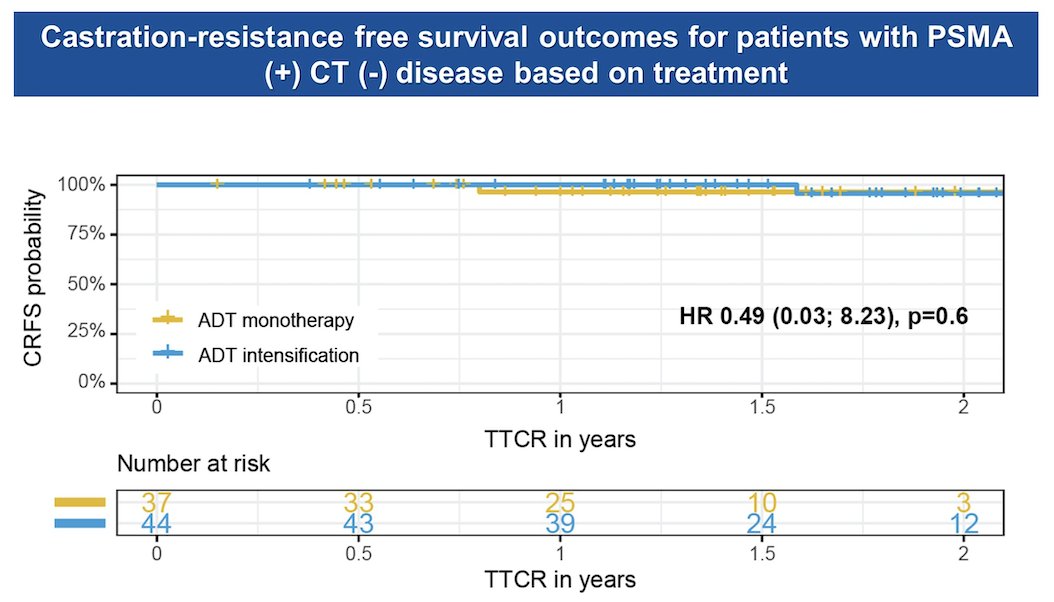
In the closing remarks, these were the following key messages:
- Patients with PSMA+CT- prostate cancer compared to patients with PSMA+/CT+ PC have better prognosis and outcomes in this retrospective cohort
- Low rates of PSA progression and longer time to castration resistance was observed in patients with PSMA+CT-
- Prospective trials such as EA8191/INDICATE (NCT04423211) are ongoing to evaluate the utility of ADT intensification in this new molecular stage of the disease (PSMA+CT)
Presented by: Wadih Issa, MD, Postdoctoral GU Oncology Research Fellow, Division of Hematology and Oncology, Department of Internal Medicine, University of Texas Southwestern, Dallas, TX
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31st and June 4th.


