(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured a session on prostate cancer, and a presentation by Dr. Stephen Freedland discussing a post hoc analysis of EMBARK assessing sexual activity patient-reported outcomes in patients who were sexually active or interested in sex at baseline.
After primary definitive therapies including radical prostatectomy and/or radiotherapy, about one-third of patients with non-metastatic hormone-sensitive prostate cancer experience biochemical recurrence within 10 years. In EMBARK, enzalutamide + leuprolide or enzalutamide monotherapy delayed metastasis-free survival versus placebo + leuprolide while maintaining high quality of life in high-risk biochemically recurrent nonmetastatic hormone-sensitive prostate cancer.1
The EMBARK prespecified patient-reported outcomes analyses demonstrated that enzalutamide combination and monotherapy maintained health-related quality of life in patients versus leuprolide alone.2 Sexual activity (a composite score from European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Prostate 25 [QLQ-PR25]) was better preserved with enzalutamide monotherapy versus placebo + leuprolide with no difference between enzalutamide + leuprolide versus placebo + leuprolide. To better understand effect on sexual activity in relevant subgroups, Dr. Freedland and colleagues examined sexual activity in patients who were sexually active or interested in sex at baseline.
The EMBARK study design is as follows:
The impact of treatment on sexual activity was assessed in two subgroups of patients: those who reported being interested in sex at baseline and those who reported being sexually active at baseline: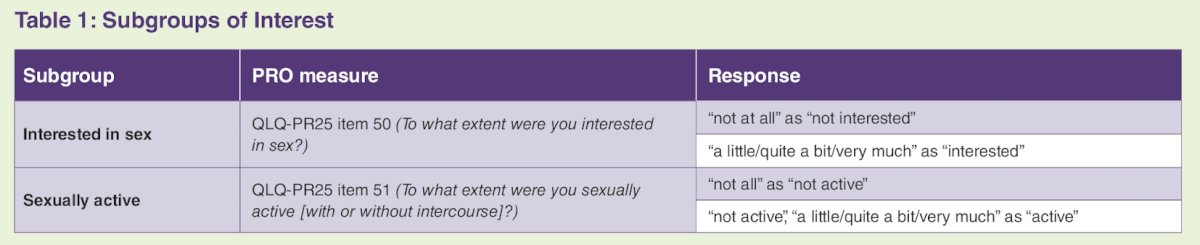
Quality of life was assessed (baseline, every 12 weeks) until metastasis/death. Cox regression was used to examine time to confirmed deterioration (confirmed at next visit) defined by one category change for sexual interest, activity, satisfaction, erectile function, and feeling like a man using QLQ-PR25 and FACT-P items: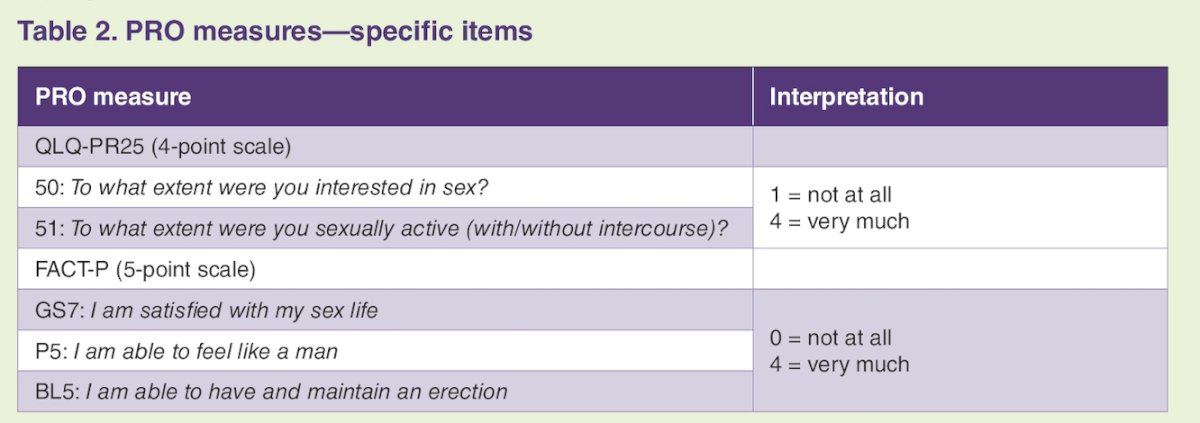
These analyses intend to treat the population.
Among patients interested in sex (n = 694): enzalutamide monotherapy (n = 225, 32%), enzalutamide combination (n = 245, 35%), and leuprolide alone (n = 224, 32%). Time to confirmed deterioration was delayed with enzalutamide monotherapy versus leuprolide alone in terms of QLQ-PR25 sexual activity domain, sexual interest, sexual activity, sexual satisfaction, and erectile function. In contrast, time to confirmed deterioration in erectile function was shorter with enzalutamide + leuprolide versus placebo + leuprolide: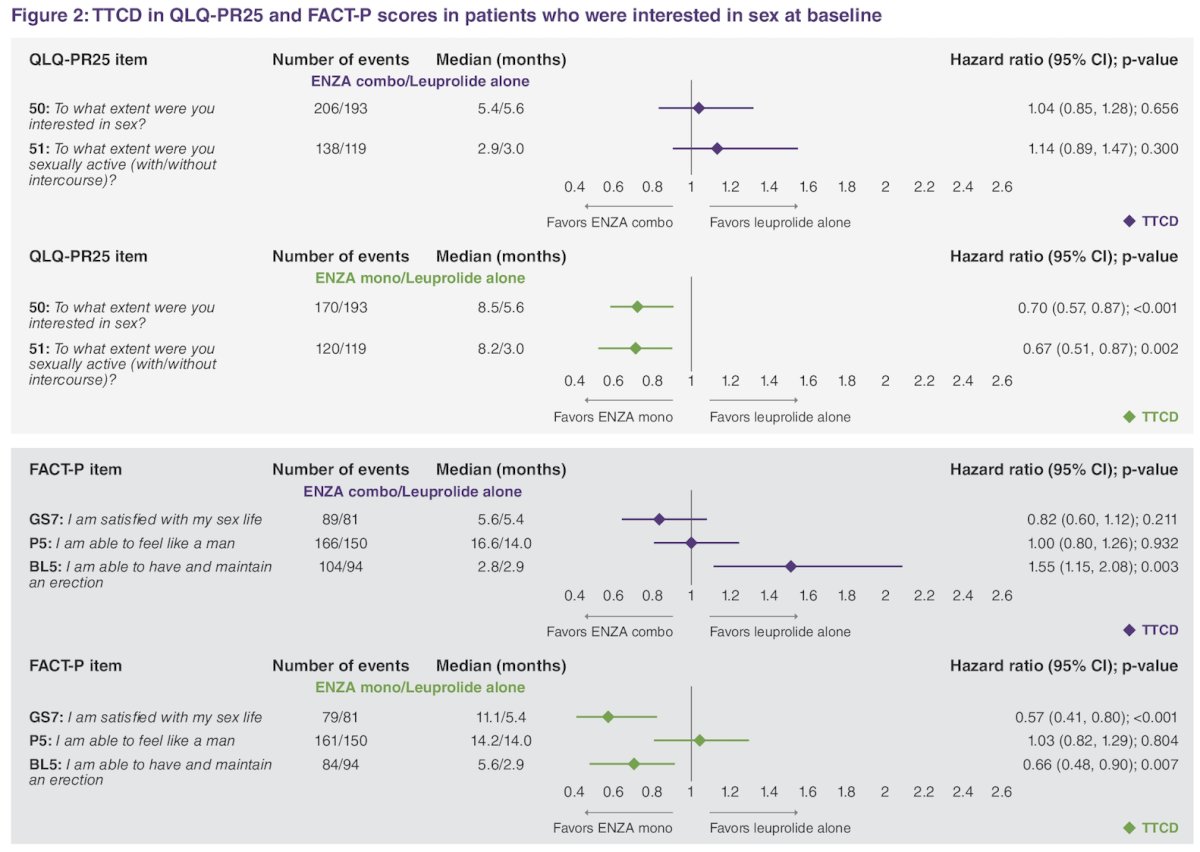
At baseline, 437 patients were sexually active: enzalutamide monotherapy (n = 149, 34%), enzalutamide combination (n = 153, 35%), and leuprolide alone (n = 135, 31%). Time to confirmed deterioration was delayed with enzalutamide monotherapy versus leuprolide alone in terms of QLQ-PR25 sexual activity domain interest in sex, extent of sexual activity, satisfaction with sex life, and erectile dysfunction. Time to confirmed deterioration in erectile function was shorter with enzalutamide + leuprolide versus placebo + leuprolide: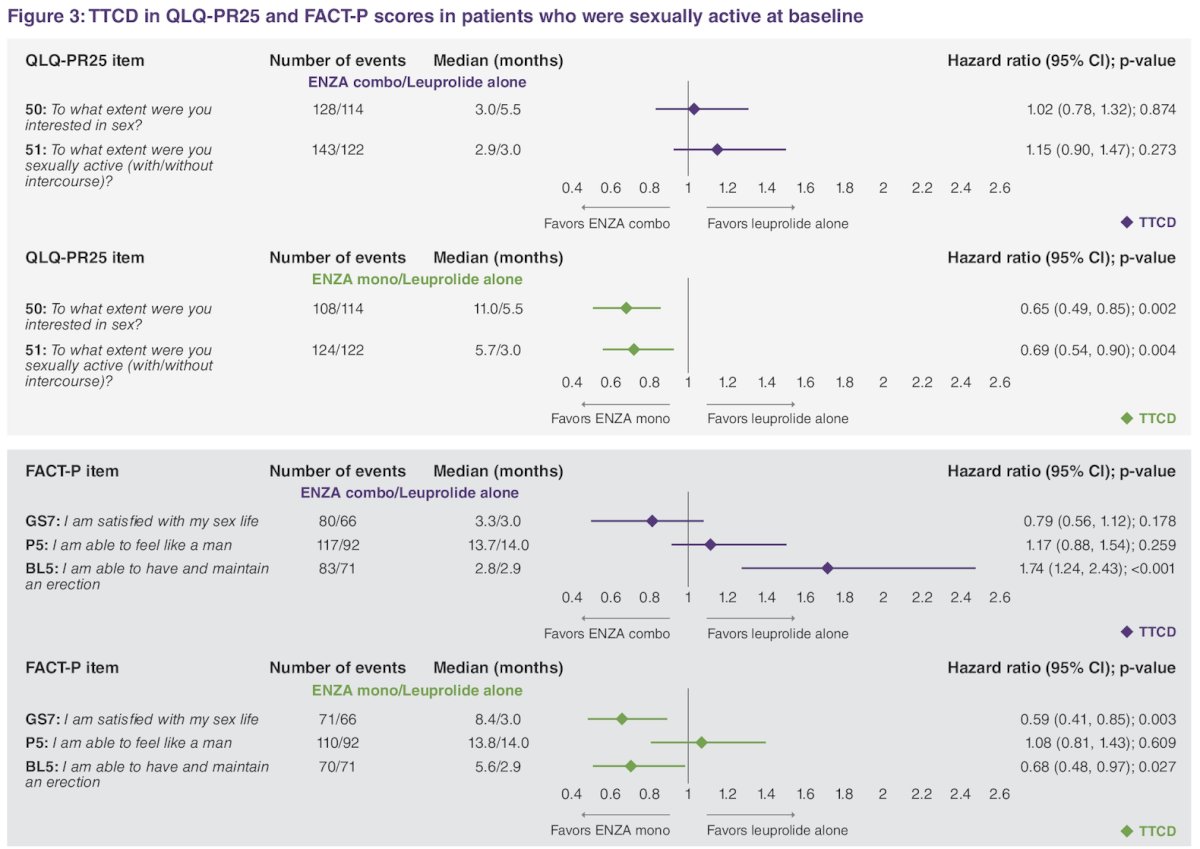
Dr. Freedland concluded his presentation discussing a post hoc analysis of EMBARK assessing sexual activity patient-reported outcomes in patients who were sexually active or interested in sex at baseline with the following take-home messages:
- Among patients who were interested in sex or sexually active at baseline, enzalutamide monotherapy better preserved sexual activity versus placebo + leuprolide in terms of sexual activity domain, interest, activity, satisfaction, and maintaining erection
- Adding enzalutamide to leuprolide had no impact on interest, activity, or satisfaction but may adversely affect erectile function, though differences in the median time to confirmed deterioration were clinically non-significant (0.06 months [1.8 days] and 0.09 months [2.7 days] in patients interested in sex and sexually active at baseline, respectively)
- The metastasis-free survival data, preserved health-related quality of life with no new safety signals, and better preserved sexual activity support the use of enzalutamide monotherapy in patients with high-risk biochemical recurrence non-metastatic hormone-sensitive prostate cancer
Presented by: Stephen J. Freedland, MD, Director of the Center for Integrated Research in Cancer and Lifestyle, Associate Director for Training and Education at the Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinari, Los Angeles, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
References:
- Freedland SJ, de Almeida Luz M, De Giorgi U, et al. Improved Outcomes with Enzalutamide in Biochemically Recurrent Prostate Cancer. N Engl J Med 2023 Oct 19;389(16):1453-1465.
- Freedland SJ, Gleave M, De Giorgi U, et al. Enzalutamide and Quality of Life in Biochemically Recurrent Prostate Cancer. NEJM Evid. 2023 Dec;2(12):EVIDoa2300251.


