(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago, IL was host to a prostate, testicular, and penile cancers poster session. Dr. Steven Yip presented the results of an ad hoc analysis of TALAPRO-2 evaluating circulating tumor cell conversion and CTC0 as prognostic biomarkers for efficacy in metastatic castration-resistant prostate cancer (mCRPC) patients receiving talazoparib + enzalutamide versus placebo + enzalutamide as first-line treatment.
In TALAPRO-2 (NCT03395197), talazoparib plus enzalutamide significantly improved radiographic progression-free survival (rPFS) compared to placebo plus enzalutamide as first-line treatment for mCRPC unselected for homologous recombination repair (HRR) gene alterations (median not reached versus 21.9 months; hazard ratio [HR]=0.63; 95% confidence interval [CI]: 0.51–0.78; p<0.0001).1
In a prior analysis of five randomized phase 3 trials in patients with mCRPC, circulating tumor cell (CTC) reduction from ≥5 to <5 per 7.5 mL of blood (CTC conversion) or from ≥1 to 0 (CTCO) at Week 13 was prognostic for overall survival, with higher discriminatory power than PSA reduction.2 In this study, Dr. Yip and colleagues examined CTC conversion and CTC0 as candidate prognostic biomarkers for rPFS in TALAPRO-2.
Blood was serially collected at screening, Weeks 1, 9, 17, and 25, and safety follow-up visits. Samples were shipped in real time for CTC enumeration using CELLSEARCH (Menarini Silicon Biosystems) at a central laboratory (Covance). Baseline CTC counts were based on Week 1 results (available screening results were used if Week 1 results were unavailable). For post-baseline calculations, the CTC evaluable population was defined as all patients from the safety population with a baseline CTC assessment and at least one post-baseline CTC assessment. CTC reductions were assessed as reductions from ≥5 to <5 per 7.5 mL of blood (CTC conversion) or from ≥1 to 0 (CTCO). The data cutoff date was August 16, 2022.
At baseline, 653 patients in the intent-to-treat population were evaluable for CTC counts: 213/653 (33%) had CTC counts ≥5 per 7.5 mL of blood, and 353/653 (54%) had CTC counts ≥1.
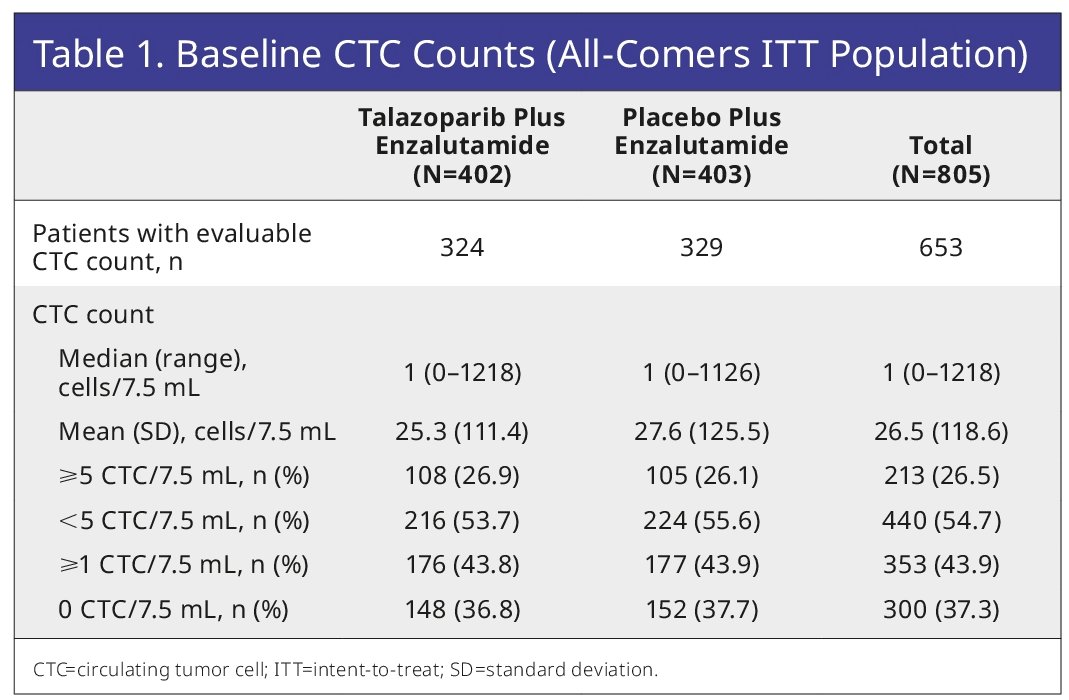
The CTC conversion rate and CTC0 were numerically higher for talazoparib plus enzalutamide (80% and 72%, respectively), versus placebo plus enzalutamide (66% and 61%).
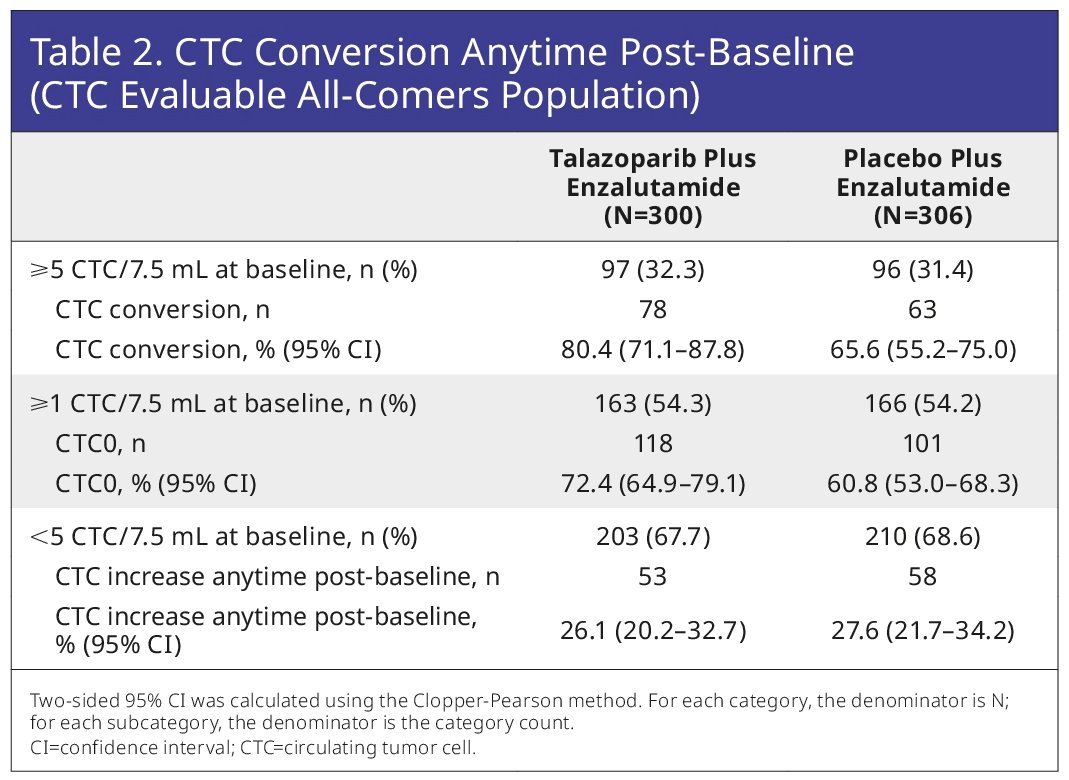
For patients with CTCs <5 at baseline, the fractions showing any increase post-baseline were low and similar across treatment arms (26–28%; Table 2). At Week 9, 144 patients were evaluable for CTC conversion (talazoparib plus enzalutamide, n=71; placebo plus enzalutamide, n=73), and 254 were evaluable for CTC0 (talazoparib plus enzalutamide, n=119; placebo plus enzalutamide, n=135). CTC conversion at Week 9 was prognostic for rPFS benefit for talazoparib plus enzalutamide (HR=0.13; 95% CI: 0.06–0.32; p<0.0001) and placebo plus enzalutamide (HR=0.16; 95% CI: 0.07–0.40; p<0.0001).
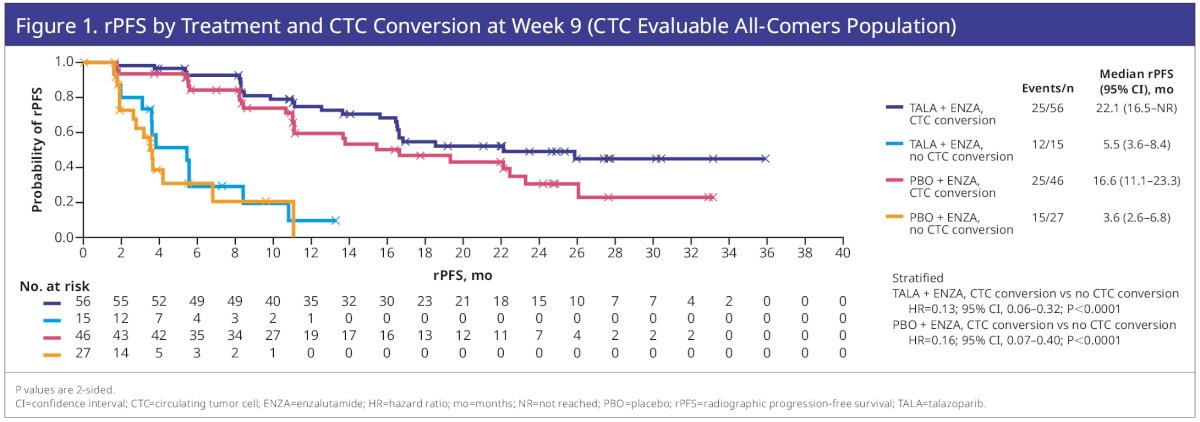
Similarly, CTC0 at Week 9 was prognostic for talazoparib plus enzalutamide (HR=0.33; 95% CI: 0.19–0.57; p<0.0001) and placebo plus enzalutamide (HR=0.41; 95% CI: 0.24–0.69; p=0.0006).

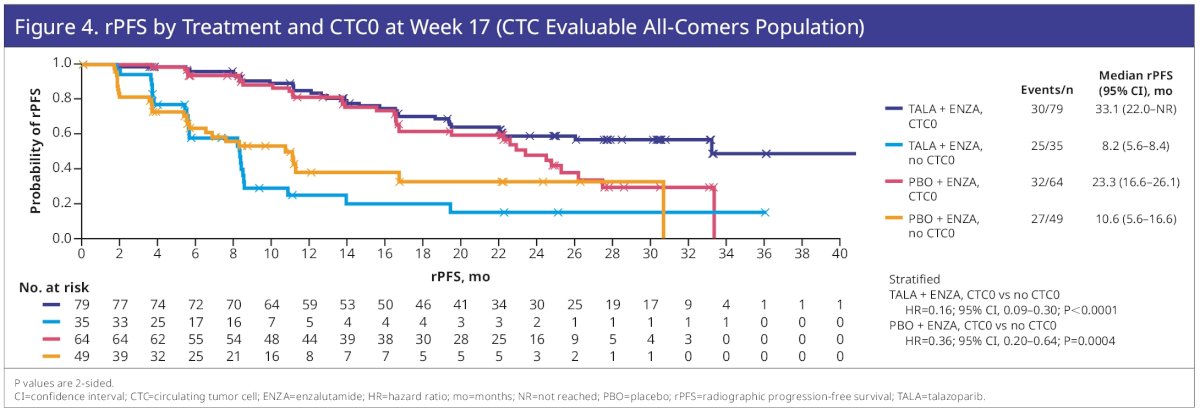
At Week 17, 132 patients were evaluable for CTC conversion (talazoparib plus enzalutamide, 64; placebo plus enzalutamide, 68), with 227 evaluable for CTC0 (talazoparib plus enzalutamide, 114; placebo plus enzalutamide, 113). CTC conversion at Week 17 was prognostic for rPFS benefit for talazoparib plus enzalutamide (HR=0.28; 95% CI: 0.10–0.73; p=0.0065) and for placebo plus enzalutamide (HR=0.26; 95% CI: 0.11–0.59; p=0.0006).

CTC0 at Week 17 was also prognostic for talazoparib plus enzalutamide (HR=0.16; 95% CI: 0.09–0.30; p<0.0001) and for placebo plus enzalutamide (HR=0.36; 95% CI: 0.20–0.64; p=0.0004).
Dr. Yip concluded as follows:
- In TALAPRO-2, CTC conversion rate and CTC0 were numerically higher for patients who received talazoparib plus enzalutamide (80% and 72%, respectively) versus those who received placebo plus enzalutamide (66% and 61%).
- CTC reduction at Week 9 and 17 was prognostic of improved rPFS in both treatments.
- This is the first time such an association has been demonstrated in a phase 3 trial featuring a poly(ADP-ribose) polymerase (PARP) inhibitor.
- This analysis was limited because not all regions supported CTC collection. Missing results mainly reflected technical and logistical limitations and challenges.
- These results support the broad prognostic utility of CTC enumeration in mCRPC, particularly in the context of PARP inhibitor therapy.
Presented by: Steven Yip, MD, MSc, Clinical Associate Professor, Medical Oncologist, Tom Baker Cancer Centre, Cumming School of Medicine, Calgary, AB
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31st and June 4th
References:
- Agarwal N, Azad AA, Carles J, et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): A randomized, placebo-controlled, phase 3 trial. Lancet. 2023 Jul 22;402(10398):291-303.
- Heller G, McCormack R, Kheoh T, et al. Circulating Tumor Cell Number as a Response Measure of Prolonged Survival for Metastatic Castration-Resistant Prostate Cancer: A Comparison With Prostate-Specific Antigen Across Five Randomized Phase III Clinical Trials. J Clin Oncol. 2018;36(6): 572-80.


