(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured a session on prostate cancer, and a presentation by Dr. Scott Tagawa discussing the final results of a phase I/II dose-escalation study of fractionated dose 177Lu-PSMA-617 for progressive metastatic castration-resistant prostate cancer (mCRPC).
Prostate cancer commonly expresses PSMA and has dose-responsive radiosensitivity, and 177Lu-based radioligands usually use an empiric dosing regimen. This first dose-escalation study of 177Lu-PSMA-617 employed a dose-dense fractionation schedule intended to allow delivery of higher total doses to overcome radioresistance due to repopulation. Previously presented at ESMO 2018, there was no dose-limiting toxicity at the highest dose. At the 2024 ASCO annual meeting, Dr. Tagawa and colleagues reported mature results of the study.
Patients were included if they had progressive mCRPC following more than one potent androgen receptor pathway inhibitor (ie. abiraterone/enzalutamide) and chemotherapy (or unfit/refuse taxane), without a limit on number of prior therapies, adequate organ function, and an ECOG performance status of 0-2. Treatment included a single cycle of fractionated dose 177Lu-PSMA-617 on day 1 and day 15. In phase I dose-escalation, patients received one cycle of 7.4 - 22 GBq followed by Simon 2-stage phase II at 22.2 GBq. PSMA expression was not required for treatment, but pre-and post-treatment 68Ga-PSMA11 PET/CT and/or 177Lu-PSMA-617 SPECT were performed. The primary efficacy was PSA changes, and secondary outcomes included changes in CT/bone scans, PSMA PET, circulating tumor cell counts, plasma DNA, and survival.
Between January 2017 – February 2021, 50 patients treated with a median PSA of 164.3 ng/mL (range: 4.4-5541) and 35 (70%) patients were CALGB poor risk. Disease distribution included 47 (94%) patients with bone metastasis, 38 (76%) with nodal disease, 12 (24%) with lung metastasis, and 5 (10%) patients with liver metastasis (4 additional patients with PSMA PET+ liver metastasis that were not apparent on CT). Overall, 31 (62%) patients were treated with more than two prior androgen receptor pathway inhibitors, 29 (58%) patients with more than one chemotherapy regimen, 14 (28%) patients with radium-223, and 2 (4%) patients with 177Lu-J591: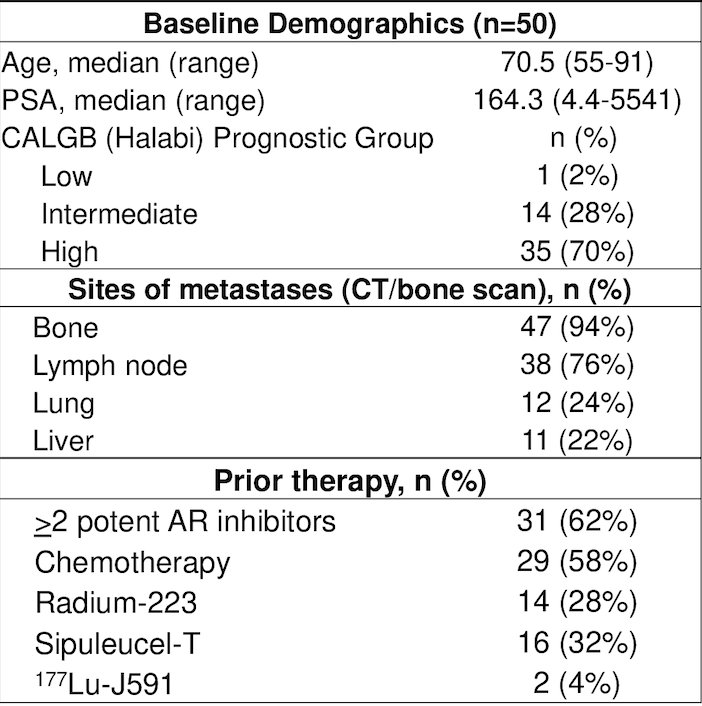
There were 27 (54%) patients treated at the dose of 22 GBq, with no dose-limiting toxicity noted during phase I. Overall, 38 (76%) patients had any PSA decline and 27 (54%) patients had a >50% PSA decline: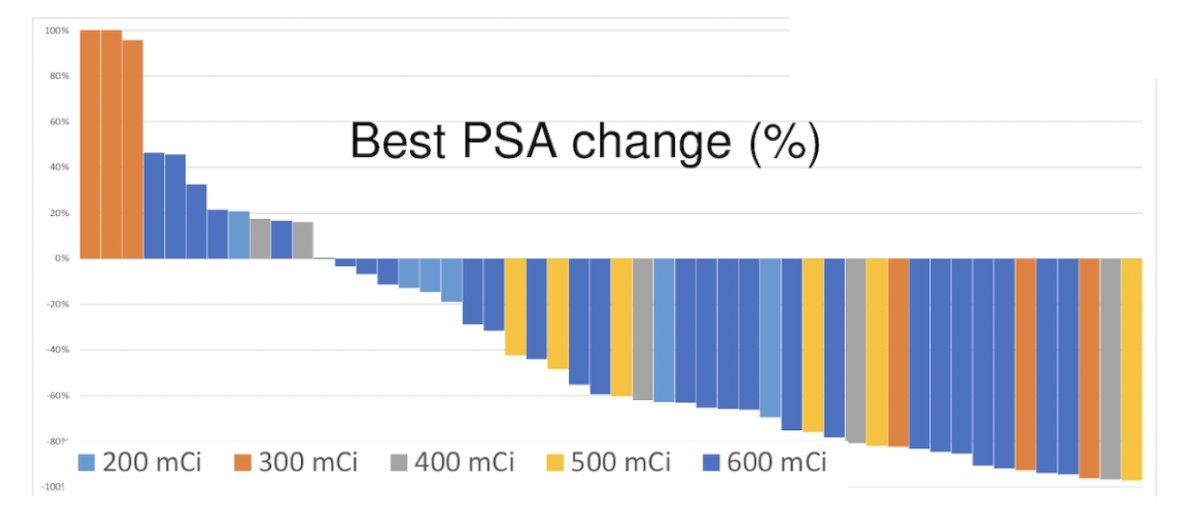
The median radiographic progression-free survival 8.3 months (95% CI 5.6-16.7), and the median overall survival was 15.7 months (95% CI 10.9-20.6). Among 17 patients with RECIST measurable disease, 6 (35.3%) patients responded, 7 (41.2%) patients were stable, and 4 (23.5%) patients progressed. Among 31 patients with paired circulating tumor cell counts, 16 (52%) patients decreased, 5 (16%) patients were stable, and 10 (32.3%) patients converted to favorable/undetectable: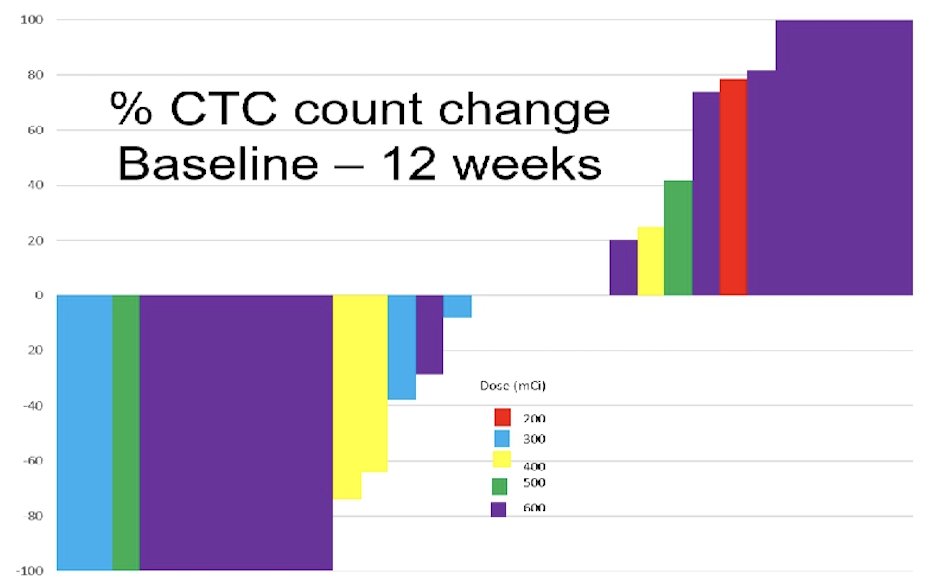
Plasma DNA with high allele-specific ploidy and ARcopy number gain was associated with overall survival on univariable analysis. Treatment-emergent adverse events included 32 (64%) patients with dry mouth, 22 (44%) patients with a pain flare, 22 (44%) patients with fatigue, 19 (38%) patients with nausea, 15 (30%) patients with anemia, 15 (28%) patients with thrombocytopenia, and 9 (18%) patients with neutropenia. All treatment emergent adverse events restricted to grade 1-2 except 6 (12%) patients with grade 3 anemia, 2 (4%) patients with grade 3/4 thrombocytopenia, and 1 (2%) patients with grade 3 neutropenia. Treatment-emergent adverse events incidence and grade were not related to dose: 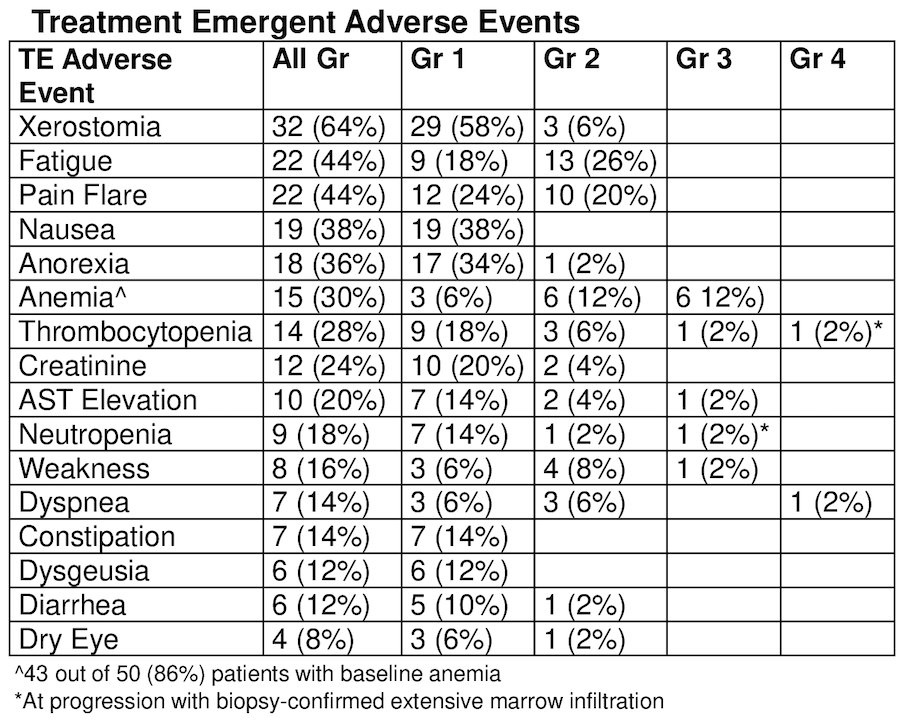
On multivariable analysis for response, only PSMA imaging score was significantly associated with PSA50 (trends for AR gain and “dose”). On multivariable analysis for overall survival, there was no variable statistically significantly associated with survival (trends for Halabi risk, prior chemotherapy, and AR gain).
Dr. Tagawa concluded his presentation discussing the final results of a phase I/II dose-escalation study of fractionated dose 177Lu-PSMA-617 for progressive mCRPC with the following take-home messages:
- The first “dose escalation” study of 177Lu-PSMA-617 demonstrates safety to 22.2 GBq in a single cycle with fractionated dosing
- In a population unselected for PSMA expression, most with post-treatment PSA decline
- Single cycle dose-intense regimen with PSA response, radiographic progression, and overall survival favorable compared to historical non-PSMA controls, as well as PSMA-selected populations with multiple, less dose-intense cycles
Presented by: Scott T. Tagawa, Professor of Medicine and Urology, Weill Cornell Medicine, New York City, New York
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
Related content: Dose-Escalation of Lutetium PSMA: Final Results from a Phase I/II Trial - Scott Tagawa


