(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago, IL between May 31st and June 4th, 2024 was host to a prostate, testicular, and penile cancers poster session. Dr. Charles Gaber presented the results of a real-world comparative analysis of the effectiveness and cardiovascular safety of enzalutamide versus abiraterone acetate amongst older men with metastatic castration-resistant prostate cancer (mCRPC).
Prostate cancer remains the second-leading cause of cancer mortality amongst U.S. men. Abiraterone and enzalutamide are androgen receptor pathway inhibitors (ARPIs) approved for treating mCRPC patients in the 1st line setting. To date, there are no randomized trials that have directly compared these two agents. There is limited literature on the comparative effectiveness and cardiovascular safety of these drugs in older men. Multimorbidity, polypharmacy, and frailty could alter the comparative outcomes for older men.
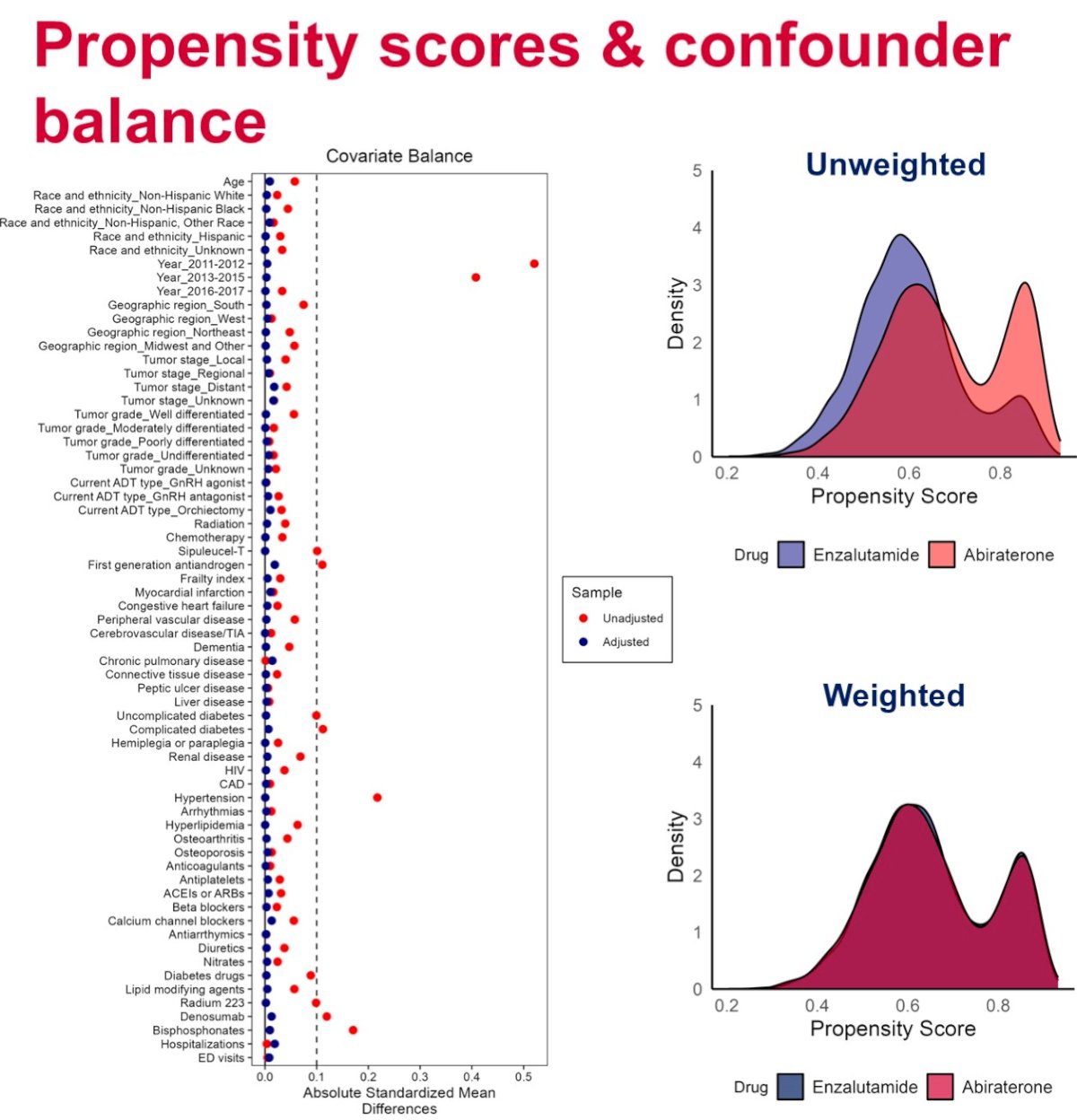
The investigators used SEER-Medicare data to identify older men (aged ≥65 years) with prostate cancer initiating abiraterone or enzalutamide between 2011 and 2017. All patients were actively receiving androgen deprivation therapy. The primary outcomes were 3-year overall survival and 1-year rate of major adverse cardiovascular event (MACE), defined as hospitalization for acute myocardial infarction, ischemic stroke, or heart failure. Inverse-probability of treatment weighting was used to adjust for demographics, tumor characteristics, comorbidities, and comedications. Kaplan Meier and Aalen-Johansen estimators were used to calculate survival and cumulative incidence rates. The results were stratified by Kim claims-based frailty index to explore heterogeneity by frailty.
The study cohort included 5,625 and 2,933 prostate cancer patients utilizing abiraterone and enzalutamide, respectively. The median patient age was 76 years. 70% of the cohort was categorized in the “frail category” of the frailty index. About one third of patients had de novo metastatic disease at diagnosis.
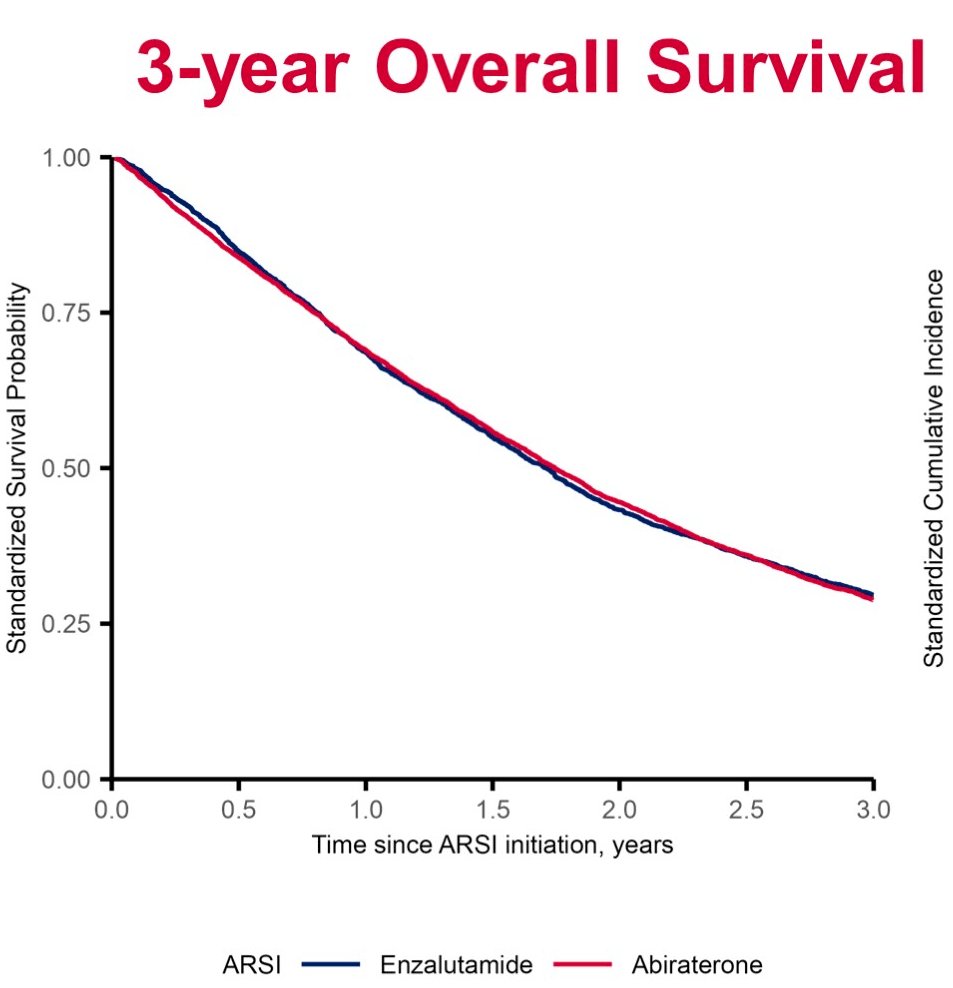
Compared to enzalutamide initiators, overall survival was similar in patients initiating abiraterone at both one-year (mortality hazard ratio (HR)=1.05; 95% CI: 0.95 – 1.17) and five-years of follow up (HR=1.01; 95% CI: 0.95 – 1.08).
The one-year rate of MACEs was higher in the abiraterone initiators (HR=1.30; 95% CI: 1.01 – 1.67).

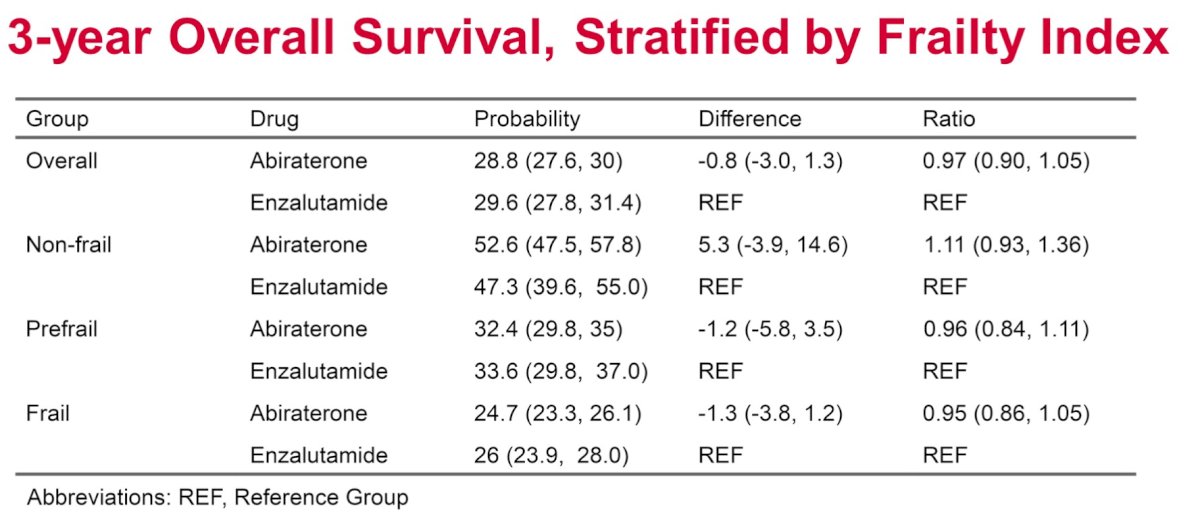
There were no significant differences in 3-year overall survival rates between abiraterone and enzalutamide users when stratified by frailty index category.
The MACE risk ratio for abiraterone versus enzalutamide was higher in frail patients (risk ratio [RR]: 1.31), compared to non-frail (RR: 0.54) and pre-frail patients (RR: 1.23).
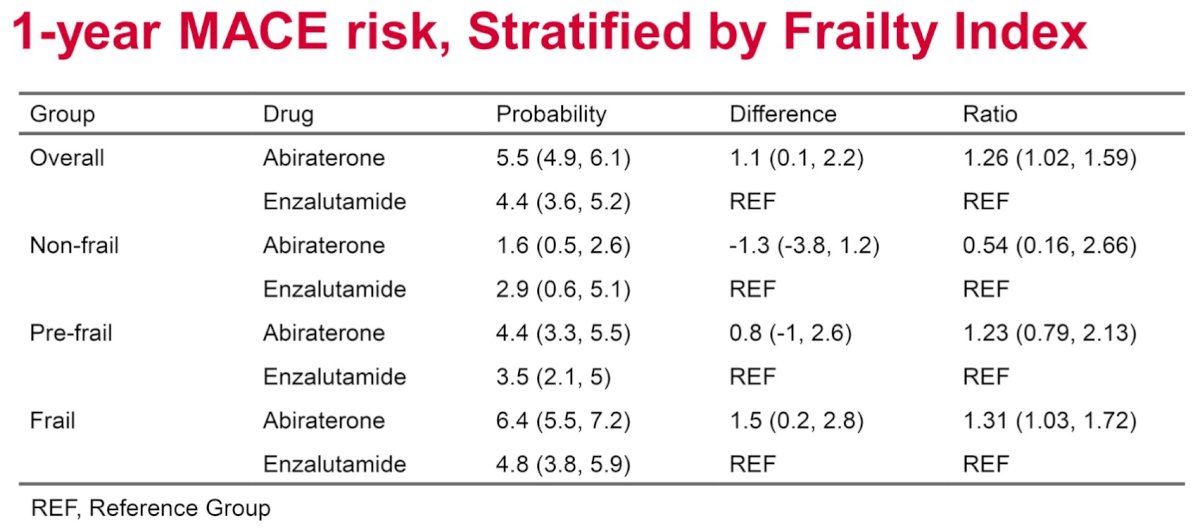
Dr. Gaber concluded that abiraterone is associated with a 26% increased rate of MACEs compared to enzalutamide in older men with mCRPC, although overall survival remains equivalent. The MACE risk ratio for abiraterone versus enzalutamide is higher in frail patients, compared to non-frail and pre-frail patients, underscoring the importance of frailty assessment when considering treatment options for older men.
Presented by: Charles Gaber, PhD, MPH, Assistant Professor, Department of Pharmacy Systems, Outcomes & Policy, University of Illinois-Chicago, Chicago, IL
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31st and June 4th


