(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago, IL between May 31st and June 4th, 2024 was host to a prostate, testicular, and penile cancers poster session. Furaha Kariburyo-Yay presented a real-world analysis of baseline treatment patterns and overall survival in metastatic castration-resistant prostate cancer (mCRPC) patients treated with olaparib in the United States.
Olaparib was approved by the U.S. Food and Drug Administration in May 2020 for patients with homologous recombination repair gene mutations (HRRm) who have progressed following treatment with enzalutamide and/or abiraterone based on the data from the PROfound trial.1 Real-world data on olaparib monotherapy use including treatment sequence and outcomes remain limited in mCRPC patients with HRRm.
The study objective was to describe the context of olaparib initiation in patients with mCRPC, particularly in relation to prior next-generation hormonal agents (NHA) and subsequent overall survival in real-world data.
This was a retrospective observational study using structured data and curated progress notes from U.S. electronic medical record (EMR) data from patients with an mCRPC diagnosis date between 2013 and 2022. The investigators utilized the ConcertAl Oncology Dataset, which consists of de-identified EMR data drawn from geographically diverse practice locations within the U.S. and are primarily community oncology practice-based (80-90%) from both rural and urban centers. Study eligibility was as follows:
- Patients with confirmed mCRPC diagnosis, age ≥21 years, treated with FANCL, PALB2, RAD51B, RAD51C, RAD51D, or RAD54L) irrespective of test type.
- Patients with diagnoses of primary cancers other than prostate cancer or non-melanoma skin cancer or who were enrolled in an interventional clinical trial on or after mCRPC diagnosis were excluded.
The index date was the start date of the earliest olaparib monotherapy treatment. Real-world time on treatment was defined as the start of earliest olaparib monotherapy to the date of olaparib monotherapy discontinuation, the day prior to the start of the subsequent regimen in patients without a documented discontinuation date, or death, whichever occurred first. Data was censored at the end of the study period. Overall survival was defined from the start of earliest olaparib monotherapy treatment until death. Patients without a documented death were censored at the end of the study period. Descriptive statistics were generated for patient characteristics and treatments. Kaplan-Meier analysis was used to estimate median time-on-treatment and overall survival from the index date.
A total of 144 mCRPC olaparib-treated patients were identified. The median follow-up time from initial mCRPC diagnosis to end of record was 25.3 months. The median follow-up time from index date to end of record was 8.1 months.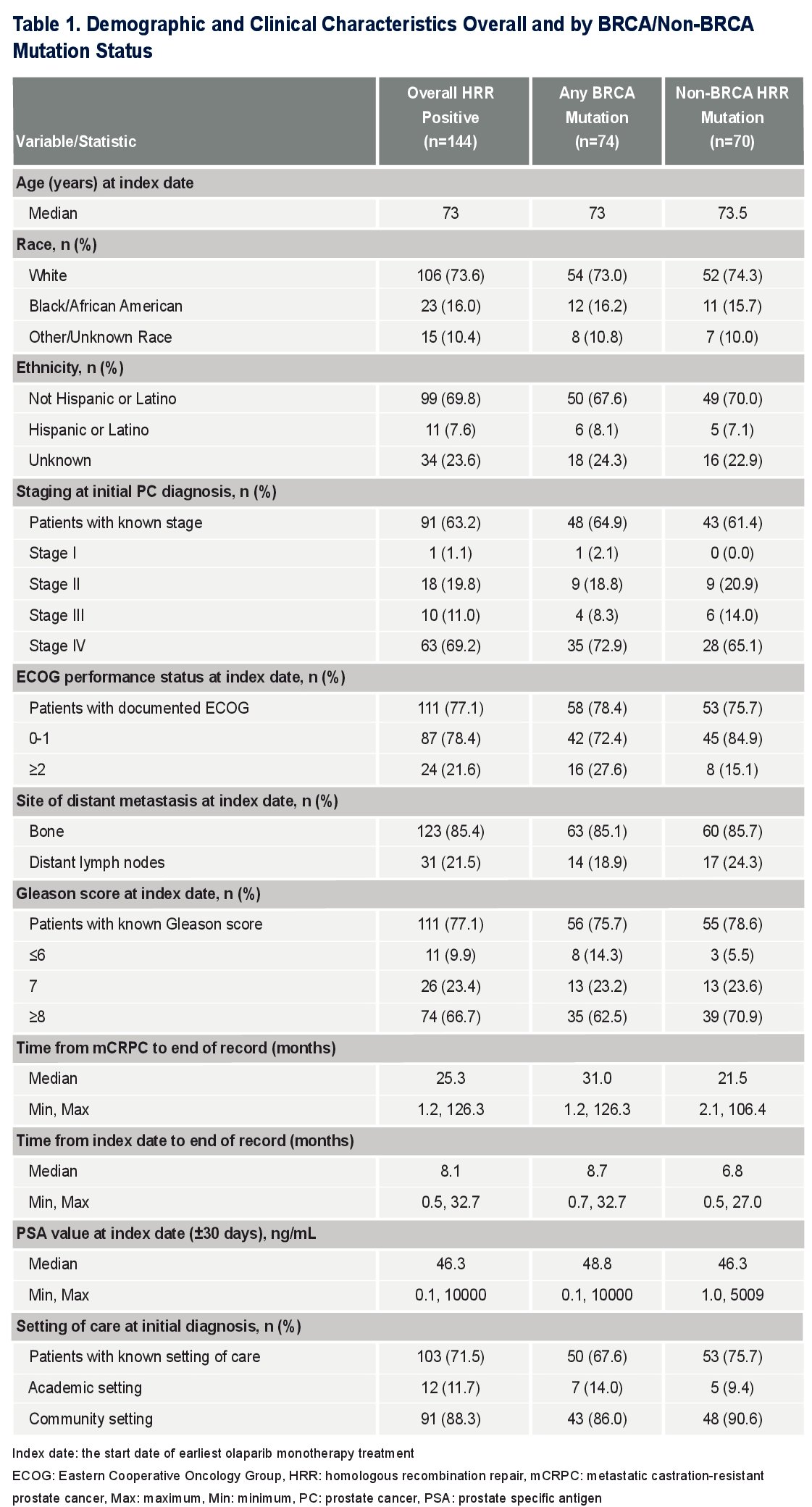
Of the 144 patients, 80.6% (116 patients) of patients progressed to mCRPC from mHSPC. Of those 116 patients, 62.1% (72 patients) received at least one NHA treatment in the mHSPC setting prior to mCRPC diagnosis. 11.8% (17 patients) of patients progressed from mCRPC.
Of those 17 patients, 41.2% (7 patients) received at least one NHA treatment in the mCRPC setting prior to mCRPC diagnosis. 7.6% (11 patients) of patients had no data prior to mCRPC diagnosis.
Overall, 82.6% initiated olaparib monotherapy in 2L+ after mCRPC diagnosis with 15.3% initiating olaparib in 5L+.
Among the 116 patients diagnosed with mHSPC before mCRPC, olaparib monotherapy was most frequently received in 2L (26.7%, 31 patients) and in 3L (22.4%, 26 patients).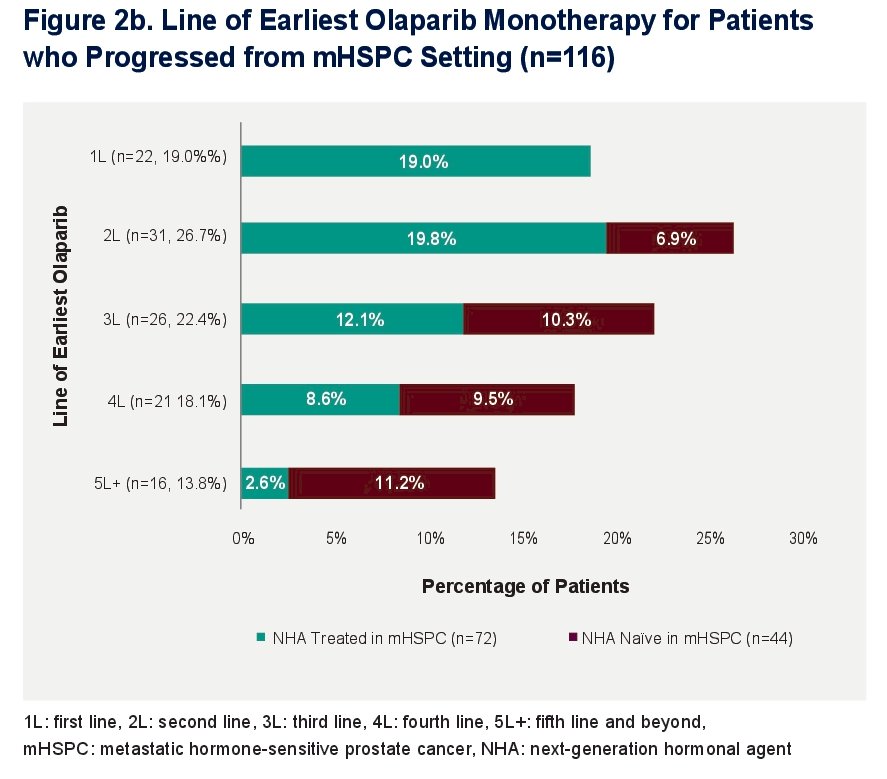
For the NHA-treated patients in the mHSPC setting, olaparib monotherapy was most frequently received in 1L (19.0%, 22 patients) and 2L (19.8%, 23 patients). Less than 3% (2.6%, 3 patients) of patients received olaparib in 5L+.
For the patients that were NHA-naive in the mHSPC setting, olaparib monotherapy was most frequently received in the 5L+ (11.2%, 13 patients).
Among the 17 patients diagnosed with nmCRPC before mCRPC, olaparib monotherapy was most frequently received in 3L (29.4%, 5 patients) and in 4L (23.5%, 4 patients). For the patients treated with NHA in the mCRPC setting, olaparib monotherapy was most frequently received in 1L (17.6%, 3 patients). For the patients that were NHA-naive in the mCRPC setting, olaparib monotherapy was most frequently received in 3L (23.5%, 4 patients).
Among the 11 patients identified at mCRPC diagnosis, olaparib monotherapy was most frequently received in 2L (45.5%, 5 patients) and 5L+ (27.3%, 3 patients).
The overall median time-on-therapy for earliest olaparib monotherapy treatment was 4.6 (95% Cl: 3.8, 6.2) months.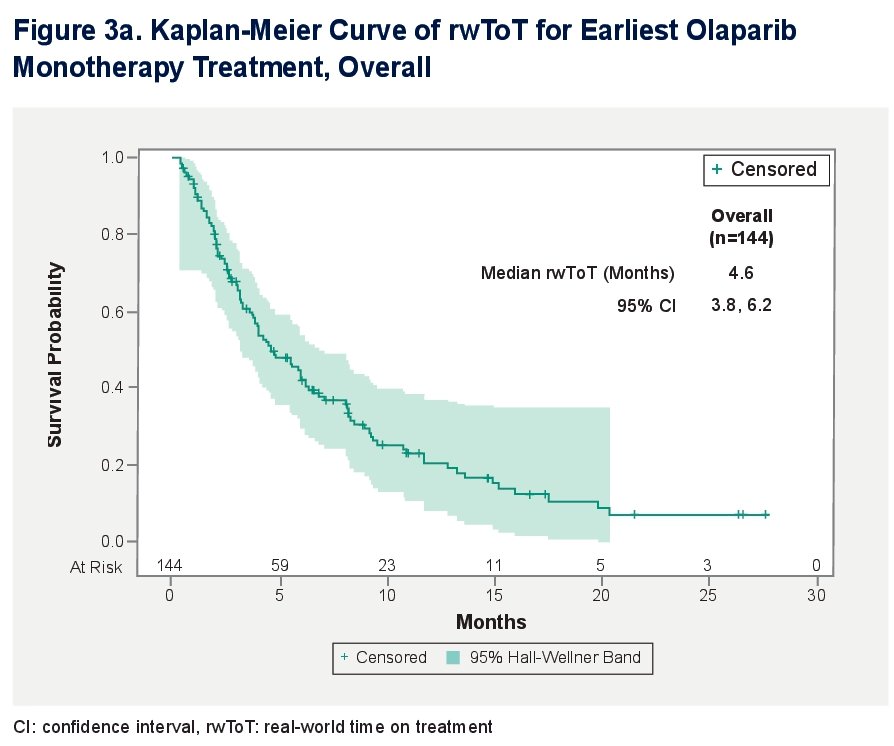
For patients with any BRCA mutation, the median time on therapy was 8.1 (95% Cl: 5.6, 9.3) months. For patients with non-BRCA HRRm, the median time on therapy was 3.2 (95% Cl: 2.5, 4.0) months.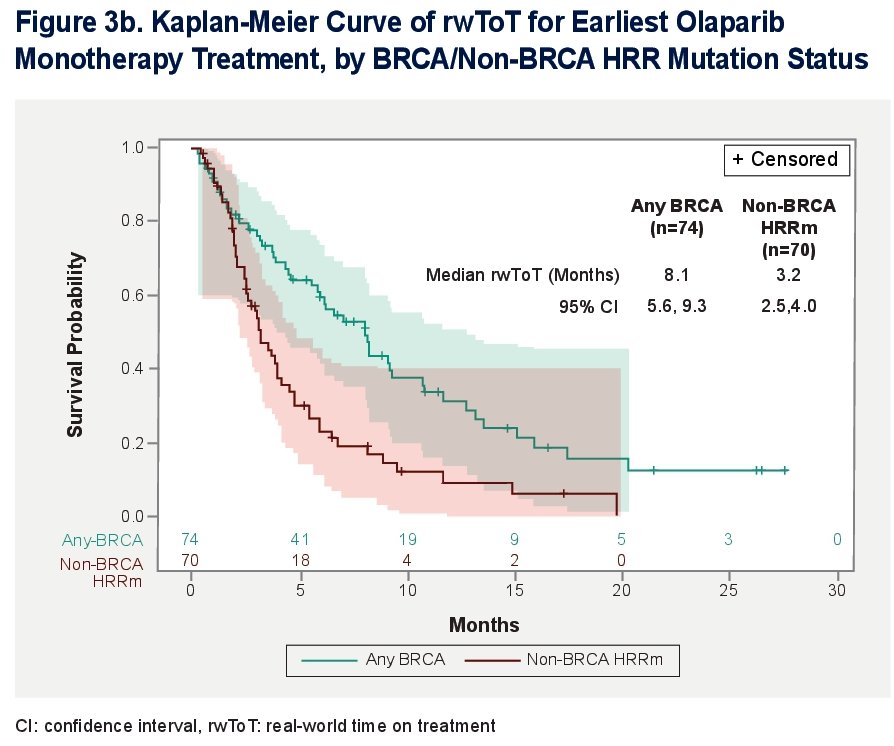
The median overall survival from earliest olaparib monotherapy was 16.5 (95% CI: 12.4, 21.2) months.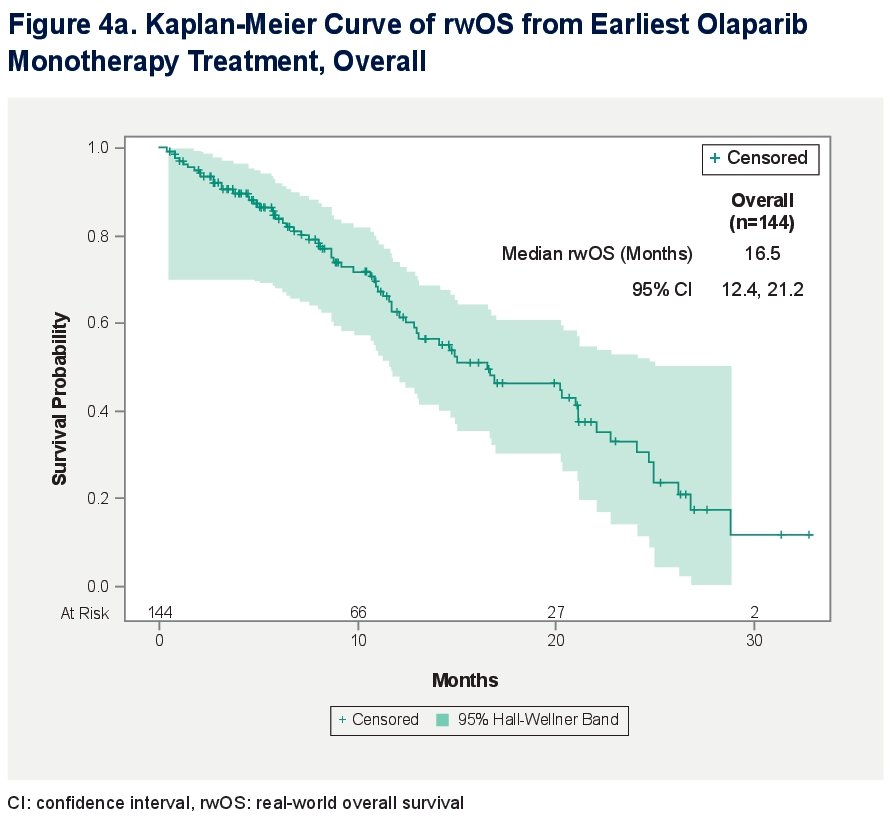
For patients with any BRCA mutation, the median overall survival was 20.3 (95% Cl: 14.7, 26.8) months. For patients with non-BRCA HRRm, the median OS was 12.9 (95% Cl: 10.9, 16.9) months.
The study investigators concluded as follows:
- This real-world analysis demonstrates that many mCRPC patients with HRRm who received olaparib are first treated in the mHSPC setting.
- Many mCRPC patients with HRRm in this study were heavily treated and received several lines of therapy in the mCRPC setting prior to receiving olaparib monotherapy.
- The real-world overall survival analysis demonstrated similar results to the PROfound clinical trial data, which demonstrates the clinical benefit of olaparib monotherapy in the real-world setting despite later line use in the real-world setting compared to PROfound.
- This analysis highlights the need for earlier treatment with olaparib monotherapy which may improve duration of therapy and real-world overall survival in patients with mCRPC whose disease has progressed after receiving an NHA.
Presented by: Furaha Kariburyo-Yay, MPH, Associate Director, Scientific at ConcertAI, Malabar, FL
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, May 31st – June 4th, 2024
References:

