(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured a session on prostate cancer, and a presentation by Dr. Edwin Posadas discussing the initial results of STEEL, intensification of ADT with enzalutamide in high-risk patients with biochemical relapse following radical prostatectomy undergoing salvage radiation. Salvage radiotherapy is an important maneuver for biochemically recurrent patients without distant metastasis, given that >30% of patients undergoing radical prostatectomy experience biochemical relapse.
Historically, patients with high-risk features who experience biochemical relapse after radical prostatectomy benefit from the addition of ADT to salvage radiotherapy. Dr. Posadas and colleagues hypothesized that intensification of androgen receptor blockade with enzalutamide would improve salvage radiotherapy outcomes for high-risk patients.
Post-prostatectomy prostate cancer patients who had biochemical relapse (PSA ≥ 0.2 ng/mL) with at least one high-risk feature (Gleason 8-10, seminal vesicle invasion, pN1, persistent PSA >0.1 ng/mL after radical prostatectomy, and PSA ≥ 0.7 ng/mL) were eligible. Patients were randomized 1:1 to 24 months of ADT with an LHRH analog or intensified ADT comprised of LHRH analog + enzalutamide. The primary endpoint was progression-free survival with progression defined as PSA ≥ 0.05 ng/mL or initiation of new therapy following salvage radiotherapy. Patients were stratified by number of aggressive features (1 versus > 1). The target accrual of 170 patients provided 80% power to detect a HR 0.65 using a one-sided log-rank test with a type I error of 0.10.
Between April 2019 and August 2022, 188 patients were enrolled. The patient characteristics were well balanced between the two arms, with a median age of 64 years, nodal involvement (pN1) present in 22%, 77% of patients with pT3a-b disease, and Gleason 9 among 52% of patients. Over 70% had > 1 aggressive feature. The median follow-up time at the time of this analysis was 15.8 months. Prostatic fossa and pelvic salvage radiotherapy were mandatory, and para-aortic radiotherapy and lymph node and prostatic fossa lesion radiotherapy boosts were left at the discretion of the radiation oncologist.
Progression-free survival favored the enzalutamide-intensified arm (HR 0.72, 80% CI: 0.56-0.94, one-sided p = 0.14). Grade 3 adverse events related to treatment in the standard versus enzalutamide arms were 11 versus 23%, while grade 4 adverse events were 4% versus 1%, respectively. There was no difference in time to grade 3+ adverse events (HR 1.55, 95% CI 0.90-2.67):
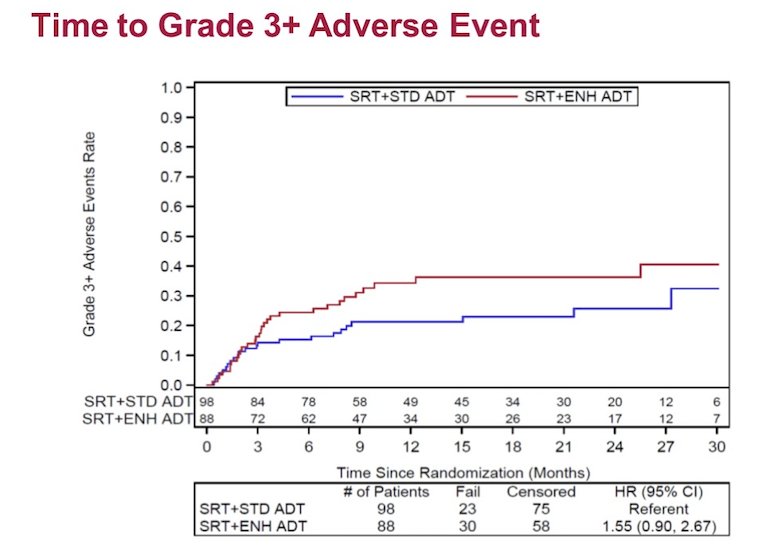
The most common adverse events (all grades, >15%) included hot flashes, fatigue, diarrhea, and decreased lymphocytes. The grade 3+ adverse events (>3%) included decreased lymphocytes and hypertension. The largest differences (>7%) in adverse events included insomnia, decreased lymphocytes. Overall, diarrhea was less frequent with enzalutamide (40% vs 54%):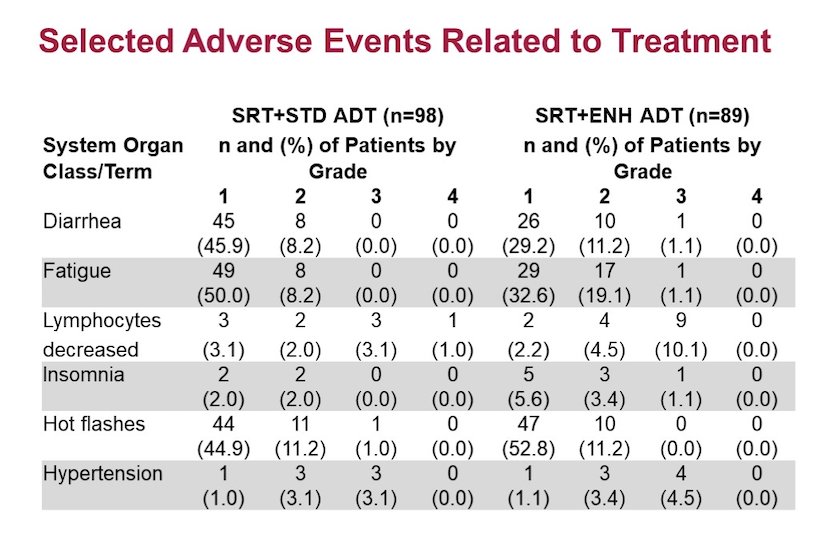
There was no difference in time to biochemical failure (HR 0.73, 95% CI 0.50-1.09) between the two groups: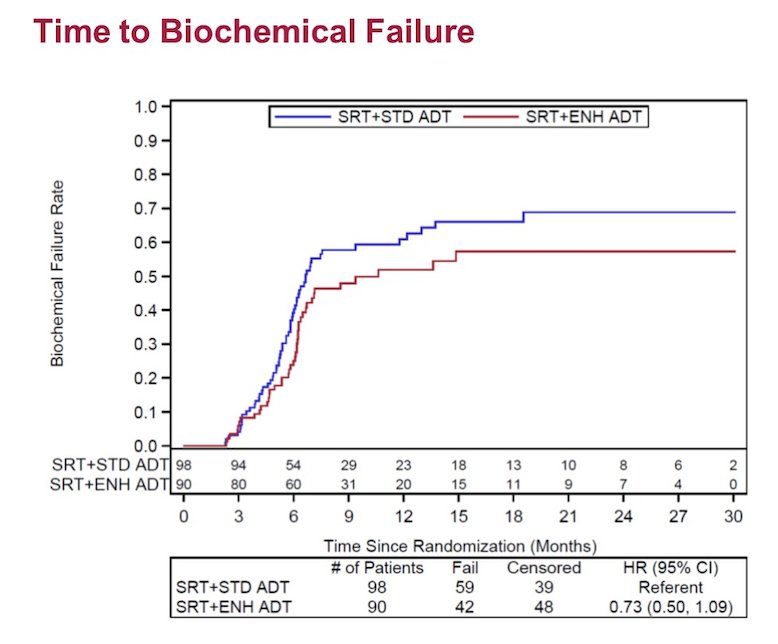
Dr. Posadas concluded his presentation discussing the initial results of STEEL, intensification of ADT with enzalutamide in high-risk patients with biochemical relapse following radical prostatectomy undergoing salvage radiation with the following take-home messages:
- The addition of enzalutamide to standard ADT did not meaningfully increase toxicity
- While there was a trend toward progression-free survival benefit from intensification, it has not yet met statistical significance based upon a new definition of PSA progression as a serum PSA concentration of 0.1 ng/mL at the time of this analysis
- Updates on progression-free survival and other clinical endpoints including quality of life will be reported with longer follow-up
Presented by: Edwin M. Posadas, MD, FACP, Director of the Experimental Therapeutics Program, Cedars-Sinai Medical Center, Los Angeles, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.


