(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured a session on prostate cancer, and a presentation by Dr. Martin Schoen discussing a comparison of outcomes with docetaxel or androgen receptor pathway inhibitor combination therapy for metastatic hormone-sensitive prostate cancer (mHSPC) by volume of disease.
Combination therapy has improved treatment of mHSPC, including ADT plus docetaxel or the androgen receptor pathway inhibitors abiraterone, enzalutamide, apalutamide, or darolutamide. However, there have been no large clinical trials that compare docetaxel to androgen receptor pathway inhibitors head to head in mHSPC. This study evaluated overall survival and time to castration resistance in patients with de novo mHSPC in the Veterans Health Administration treated with combination therapy.
Veterans were identified with initial diagnosis of ‘distant’ prostate cancer. All veterans had ADT initiated within 4 months of diagnosis and were followed until September 2023. First combination therapy with docetaxel from July 2016 – June 2021 or androgen receptor pathway inhibitor from July 2017 – June 2021 were included if initiated within 4 months after ADT. Volume of disease was determined from chart review and castration-resistance (mCRPC) by a combination of natural language processing and administrative data. Real-world progression-free survival was determined as time to mCRPC or death. Kaplan-Meier time-to-event analyses and Cox proportional hazard modeling with age, Black race, Charlson comorbidity index, PSA, body mass index, and weight change in the year prior was used for analyses.
There were 1,222 patients with de novo mHSPC identified with a median age of 71.5 years and 349 (28.6%) were Black. High-volume disease was identified in 929 (76.0%) and low volume in 293 (24.0%) of patients. Docetaxel was used in 341 (27.9%) patients and androgen receptor pathway inhibitors in 881 (72.1%) patients. Veterans with high-volume disease had shorter overall survival than low volume (23.8 vs. 64.1 months, p < 0.001). Overall, there was no difference in overall survival between docetaxel and androgen receptor pathway inhibitor (36.4 vs. 38.9 months, p = 0.68), however, docetaxel was associated with a shorter real-world progression-free survival (16.5 vs 22.1 months, p < 0.001). In high-volume disease, there was no difference in overall survival between docetaxel and androgen receptor pathway inhibitors (33.8 vs. 32.5 months, p = 0.68):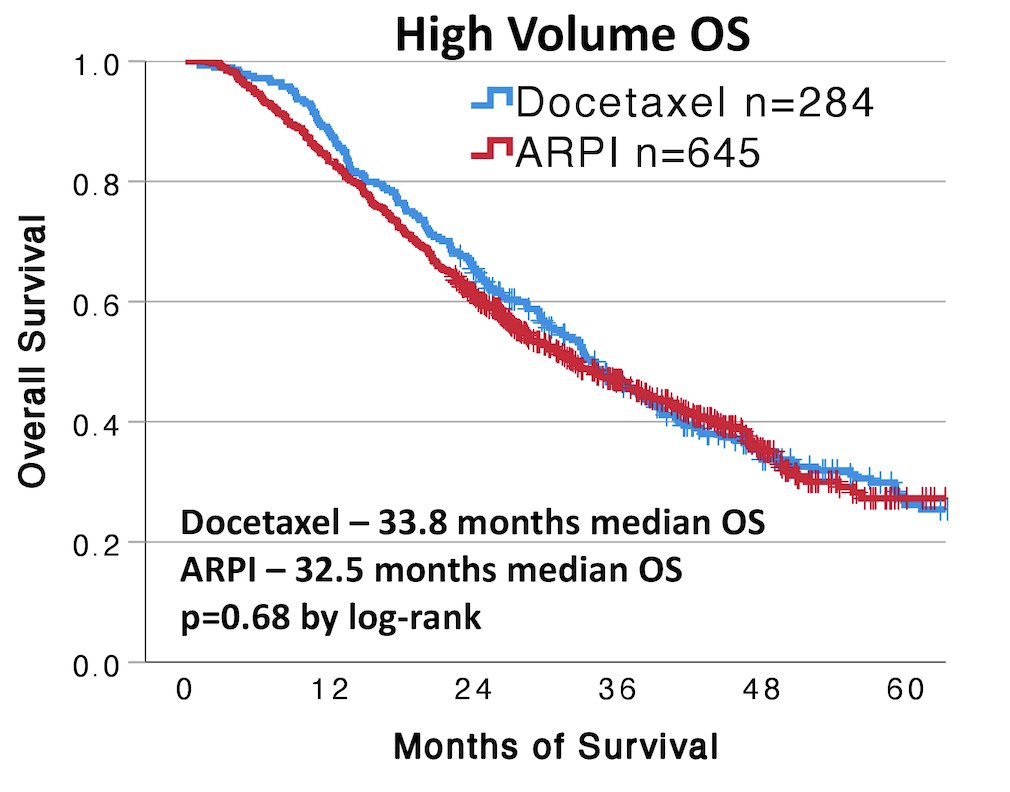
However, docetaxel was associated with a shorter real-world progression-free survival (14.9 vs 19.2 months, p = 0.002):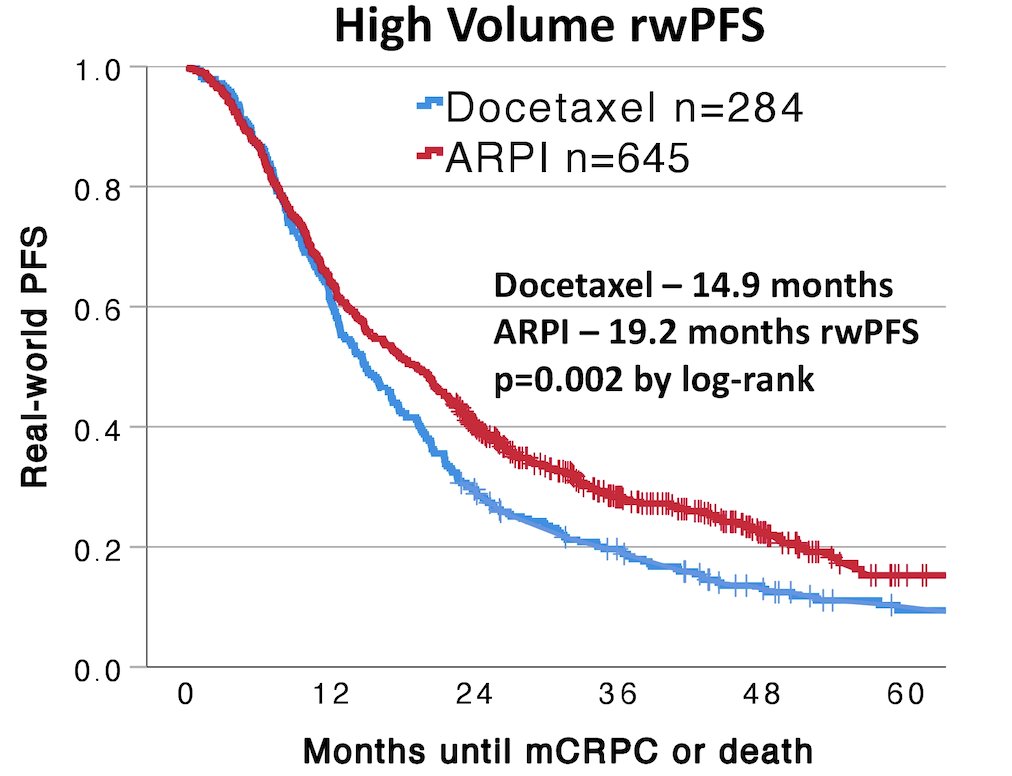
In low-volume disease, there was no difference in overall survival between docetaxel and androgen receptor pathway inhibitors (64.5 vs. 67.4 months, p = 0.75):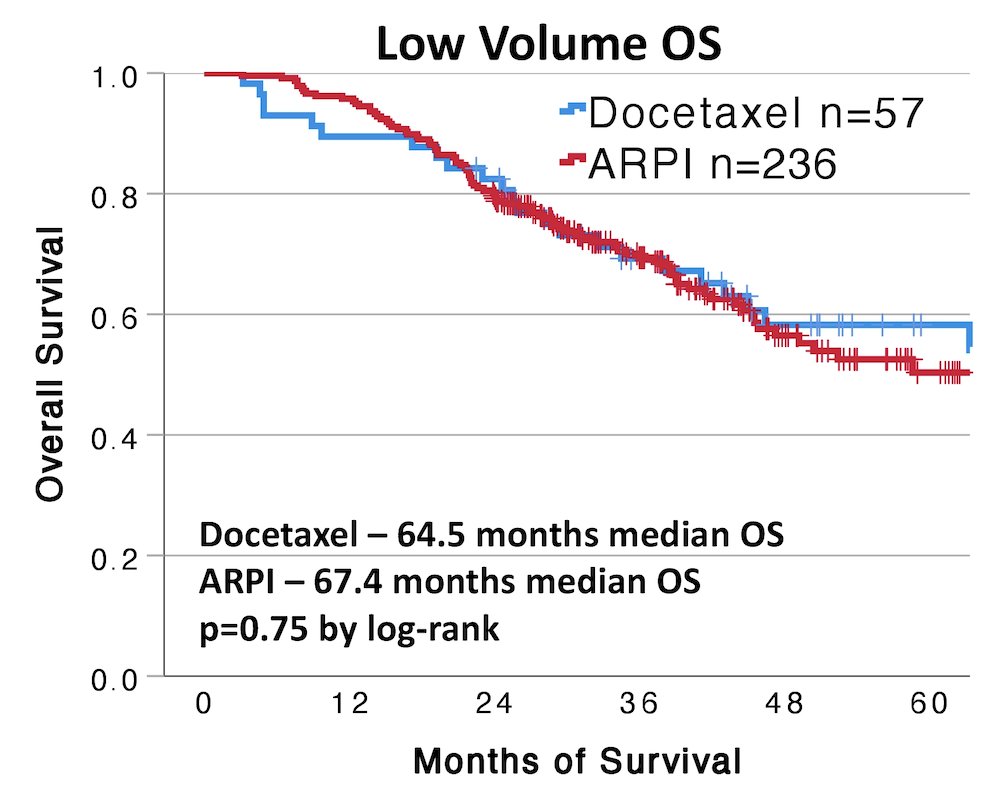
However, docetaxel was associated with a shorter real-world progression-free survival (24.3 vs 41.9 months, p = 0.001):
In a multivariable model of patients with high-volume disease, there was no difference in overall survival observed between initial treatment with docetaxel and androgen receptor pathway inhibitors (adjusted HR 0.83, 95% CI 0.69-1.00). Among low volume disease, there was also no difference in overall survival observed between initial treatment with docetaxel and androgen receptor pathway inhibitors (adjusted HR 0.81, 95% CI 0.50-1.34). Limitations include (i) Veterans with de novo mHSPC not necessarily reflecting all patients, (ii) no data on ADT + docetaxel + androgen receptor pathway inhibitor, and (iii) prostate cancer-specific mortality may be a better endpoint.
Dr. Schoen concluded his presentation discussing a comparison of outcomes with docetaxel or androgen receptor pathway inhibitors combination therapy for mHSPC by volume of disease with the following take-home messages:
- In veterans with de novo mHSPC, no difference in overall survival was observed between combination treatment with docetaxel or androgen receptor pathway inhibitors in patients with low or high-volume mHSPC
- Androgen receptor pathway inhibitors were associated with longer progression-free survival
- Due to a lack of clinical trials comparing docetaxel and androgen receptor pathway inhibitors therapy, these data may guide selection of combination therapy for mHSPC
Presented by: Martin W. Schoen, MD, MPH, Hematologist/Oncologist, Saint Louis University School of Medicine, St. Louis, MO
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.


