(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured a session on prostate cancer trials in progress, and a presentation by Dr. Adrien Holzgreve discussing the trial design of FLEX-MRT, a randomized phase 2 trial in progress of flexible and extended dosing of 177Lu-PSMA-617 molecular radioligand therapy in metastatic castration-resistant prostate cancer (mCRPC).
The FDA approved 177Lu-PSMA-617 radioligand therapy for patients with mCRPC with a fixed dosing schedule: Six cycles of 7.4 GBq administered in six-week intervals.1 However, a patient-tailored more flexible, and extended dosing schedule of 177Lu-PSMA radioligand therapy may increase treatment efficacy. No prospective data on such extended or abbreviated treatment schedule are available yet. In this randomized trial in men with mCRPC, Dr. Holzgreve and colleagues aimed to determine the efficacy of a response-based flexible dosing schedule of 177Lu-PSMA-617 radioligand therapy administered up to 12 treatment cycles compared to the current standard of care.
This is an investigator-initiated prospective phase 2, open-label, randomized, controlled, parallel-group, single-center trial. The aim is to assess the 2-year survival rate in mCRPC patients treated with a flexible dosing schedule of 177Lu-PSMA radioligand therapy up to 12 cycles in comparison to the fixed dosing schedule of 6 cycles. The trial design is as follows: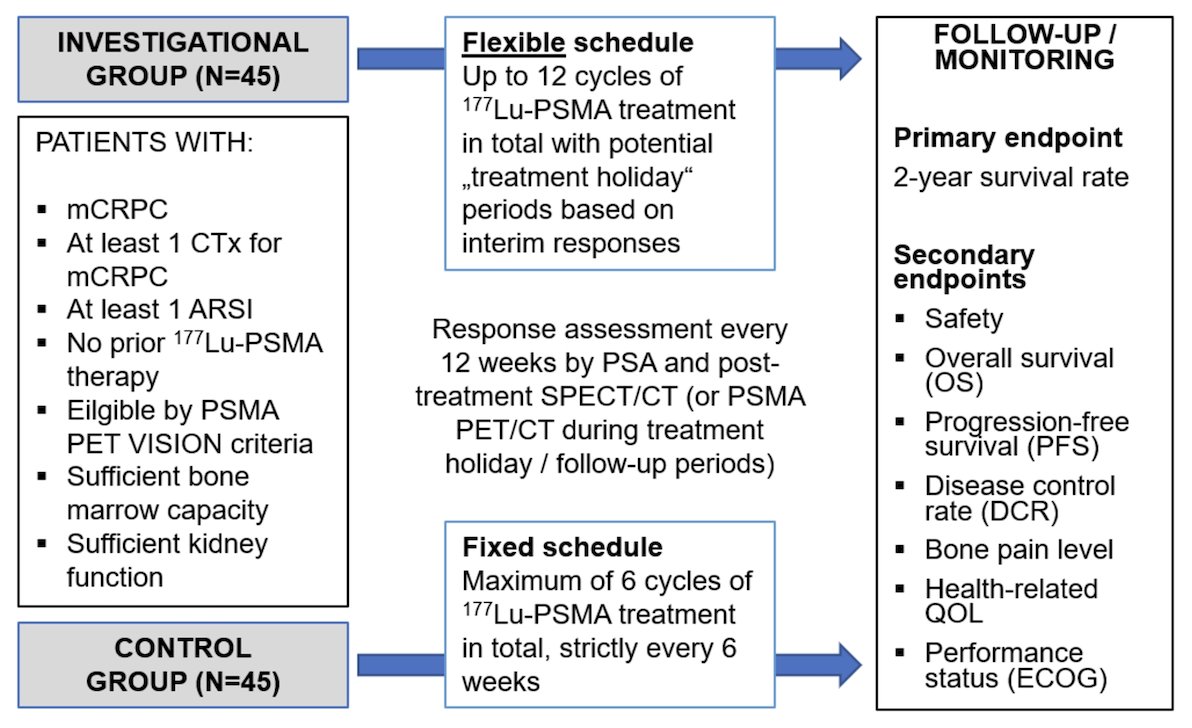
Patients with progressive mCRPC post-androgen receptor pathway inhibitor, post-taxane-based chemotherapy are eligible by PSMA PET VISION trial criteria. Exclusion criteria include prior radioligand therapy and less than 6 weeks since the last myelosuppressive therapy. The hypothesis was 2-year survival rates of 55% in the investigational group and 30% in the control group. A two-sided log-rank test with an overall sample size of 90 subjects (45 treatment group, 45 control group) achieves 80.3% power at a 0.050 significance level to detect a hazard ratio of 0.4966. Patients will be randomized in a 1:1 ratio:
- The investigational arm is treated with up to 12 cycles including potential “treatment holidays” depending on the treatment response (n = 45)
- The control arm receives 6 cycles administered in six-week intervals (n = 45)
A more detailed look of the investigational arms is highlighted below: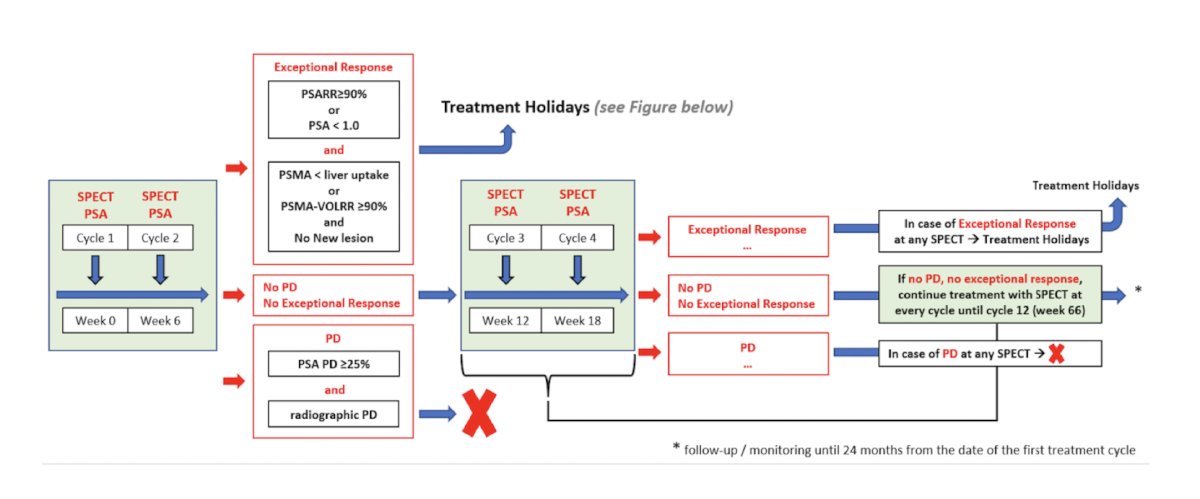
The treatment holiday details are as follows:
Imaging response to radioligand therapy is assessed using 177Lu-PSMA-617 SPECT/CT 24 hour after each cycle and PSMA PET/CT during treatment holidays (every 12 weeks), respectively. In the investigational arm, radioligand therapy will be re-started after a treatment holiday if the patient experiences a ≥25% PSA progression and an imaging progression according to the Response Evaluation Criteria in PSMA PET/CT. The primary endpoint is the 2-year survival rate calculated from the date of the first cycle of radioligand therapy. Secondary endpoints include safety by CTCAE and dosimetry, and determination of overall and progression-free survival (evidence of progression as defined by either radiographic, PSA, or clinical progression, or death from any cause).
The FLEX-MRT trial is supported by Novartis and has been approved by the FDA and the UCLA IRB. Start of enrollment was in May 2024, and the study will last for 48 months of which subject accrual (entry) occurs in the first 12 months.
Clinical trial information: NCT06216249.
Presented by: Adrien Holzgreve, MD, David Geffen School of Medicine, UCLA, Los Angeles, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
Reference:


