(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured a session on prostate cancer trials in progress, and a presentation by Dr. Stephane Oudard discussing the trial design of ALADDIN, an evaluation of darolutamide addition to ADT and radiation therapy in newly diagnosed prostate cancer with pelvic lymph nodes metastases. Standard of care for patients with prostate cancer with pelvic lymph node metastases is radiotherapy with long-term ADT.
In the STAMPEDE trial, James et al. assessed the role of abiraterone acetate with prednisolone earlier in the disease for N+M0 prostate cancer patients, showing that the addition of abiraterone acetate with prednisolone to ADT and radiotherapy significantly improved failure-free survival from 61% to 92.5%.1 Darolutamide has been previously shown to improve survival in men with castration-refractory non-metastatic prostate cancer and in mHSPC patients with the addition of ADT plus docetaxel.2-3 Dr. Oudard and colleagues hypothesized that adding darolutamide to ADT and radiotherapy could improve failure-free survival for these high-risk patients.
The ALADDIN study (NCT05116475) is a French, prospective, phase 3 trial that will enroll an estimated 152 patients with hormone-naive prostate cancer and pelvic lymph node metastases. Eligible patients are treatment-naive for hormonal therapy, with positive lymph node disease (upper limit defined as L4/L5 interspace) determined by conventional imaging or TEP choline or PSMA, ECOG performance status of 0 to 2, and adequate major organ function. Patients will be randomized by minimization 1:1 to receive ADT + intensity-modulated image-guided radiation therapy (IMRT) with darolutamide or placebo (of darolutamide). IMRT will deliver 78 Gy to the prostate, 70 Gy to the metastatic lymph nodes, and 46 Gy to the pelvic lymph node areas (2 Gy per fraction, 5 days a week) with daily cone beam CT imaging. ADT will be achieved with LHRH agonists or antagonists for 24 months, and the darolutamide regimen will be two tablets of 300 mg orally twice daily for 24 months. The trial design for ALADDIN is as follows: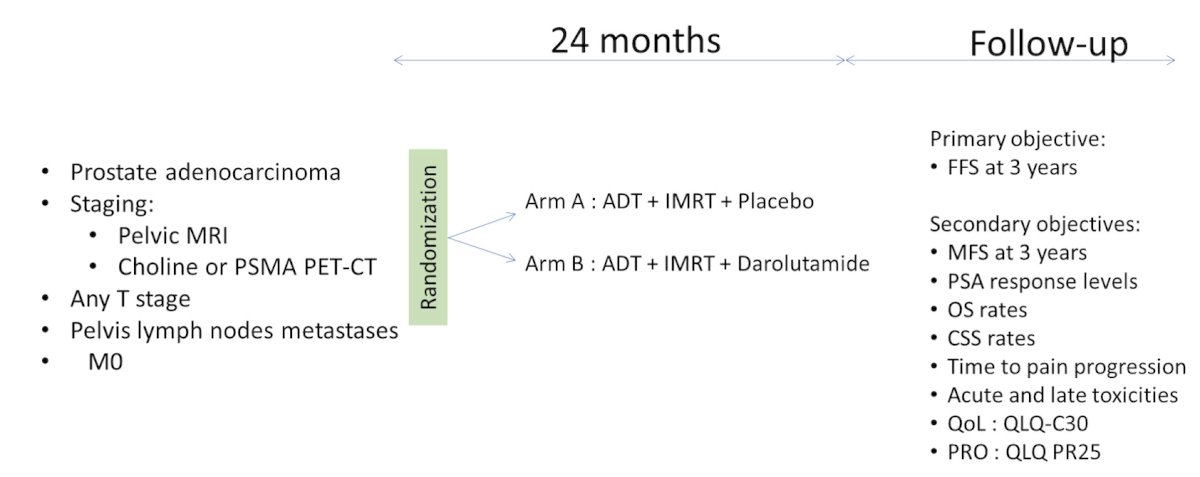
The primary endpoint is failure-free survival. Secondary endpoints will be:
- Metastasis free survival
- Progression free survival
- Safety
- Overall survival
- Health-related quality of life
A new exploratory objective is predictive modeling with artificial intelligence, whereby deep learning and convolutional neural networks will be used to identify new radiomic predictive biomarkers by analyzing CT scans of clinically node-positive prostate cancer patients treated with radiotherapy + ADT +/- darolutamide. In this study, convolutional neural networks will be trained on CT scan images from the patients included in ALADDIN to identify patterns correlating with patient responses.
Stratification factors are D'Amico risk group and sites. The planned sample size provides 80% power to detect a difference of 20% in failure-free survival (60 versus 80%) at a two-sided 0.05 significance level. The study is expected to last for 8 years, of which accrual will last for 3 years. The first participant was enrolled in the study in August 2022, and 23 centers are participating in the ALADDIN study. More than 65% of the planned patients have been included in the trial, and the DSMB convened on November 13, 2023 and allowed the study to continue as planned.
Clinical trial information: NCT05116475.
Presented by: Stephane Oudard, MD, Professor of Oncology, Georges Pompidou European Hospital, University of Paris, Paris, France
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
References:
- James ND, Spears MR, Clarke NW, et al. Failure-free survival and radiotherapy in patients with newly diagnosed nonmetastatic prostate cancer: Data from patients in the control arm of the STAMPEDE trial. JAMA Oncol. 2016 Mar;2(3):348-357.
- Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic castration-resistant prostate cancer. N Engl J Med. 2019;380(13):1235-1246.
- Smith MR, Hussain M, Saad F, et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med. 2022 Mar 24;386(12):1132-1142.


