(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago, IL was host to a prostate, testicular, and penile cancers poster session. Dr. Ruben Raychaudhuri presented the results of PLATIPARP, a phase II study of induction docetaxel plus carboplatin followed by maintenance rucaparib in metastatic castrate-resistant prostate cancer (mCRPC) patients with homologous recombination DNA repair (HRR) deficiency.
Genes involved in homologous recombination DNA repair (HRR) are inactivated in ~25% of patients with mCRPC.1 Inactivation of HRR genes has been associated with increased sensitivity to DNA damage by platinum chemotherapy and PARP inhibitors (PARPi).2,3 While both drug classes have shown efficacy, resistance and/or cumulative dose-limiting toxicities are inevitably observed. Induction therapy with platinum followed by maintenance PARPi has been effective in pancreatic and ovarian cancer. Dr. Raychaudhuri presented results from a single-center phase II trial investigating whether induction chemotherapy with docetaxel plus carboplatin followed by maintenance PARPi would provide prolonged disease control.
mCRPC patients with tumors harboring a pathogenic alteration in an HRR gene, who had received prior taxane and/or androgen receptor pathway inhibitor (ARPI), but not prior PARPi were inclusion eligible. Study patients received induction chemotherapy with 4 cycles of docetaxel 60mg/m2 with carboplatin AUC 5 IV every 21 days, followed by the PARPi rucaparib 600mg twice daily continuously as maintenance therapy until disease progression or unacceptable toxicity.
The primary endpoint was clinical/radiographic progression free survival (PFS) compared to a historical control of 9.1 months with PARPi alone without prior platinum.4 Twenty patients provided ~ 90% power to determine whether this treatment lowered risk of progression with a hazard ratio of 0.5 (implying PFS of 18.2 months), based on a 1-sided 1-sample log-rank test.
Secondary endpoints included PSA50 response rate, safety, and overall survival. Post-hoc subgroup analysis was performed for the BRCA complex group (alterations in BRCA1, BRCA2, and PALB2) and for those refractory to induction chemotherapy (no PSA decline).
Eighteen patients were enrolled between November 2018 and November 2021. Under-enrollment occurred due to loss of manufacturer support for rucaparib prior to study completion. 11/18 (61%) patients had received ≥2 prior ARPI, and 9/18 (50%) had previously received docetaxel. HRR genes included on study were:
- ATM (7), BRCA1 (3), BRCA2 (8), PALB2 (1), FANCA (1) and CHD1/SPOP (1)
- Three tumors harbored two alterations.
The median follow up was 31.5 months. 6/18 patients experienced expected ≥ grade 3 adverse events.

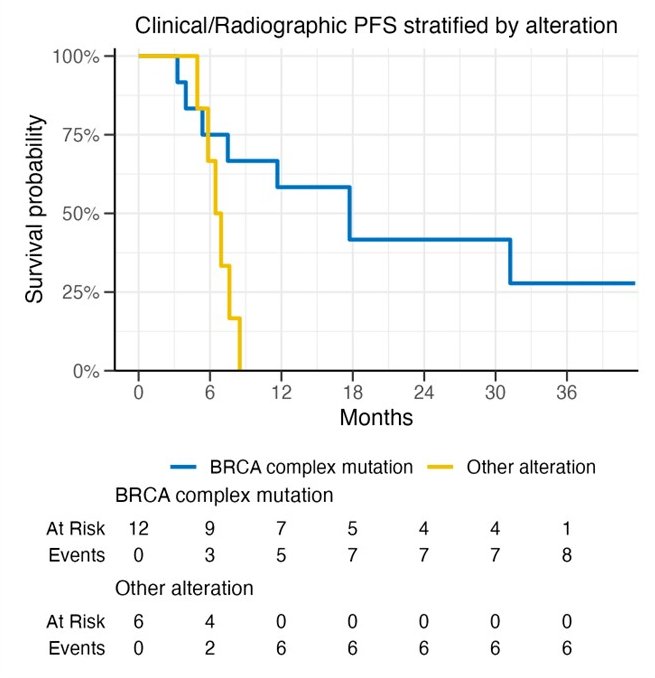
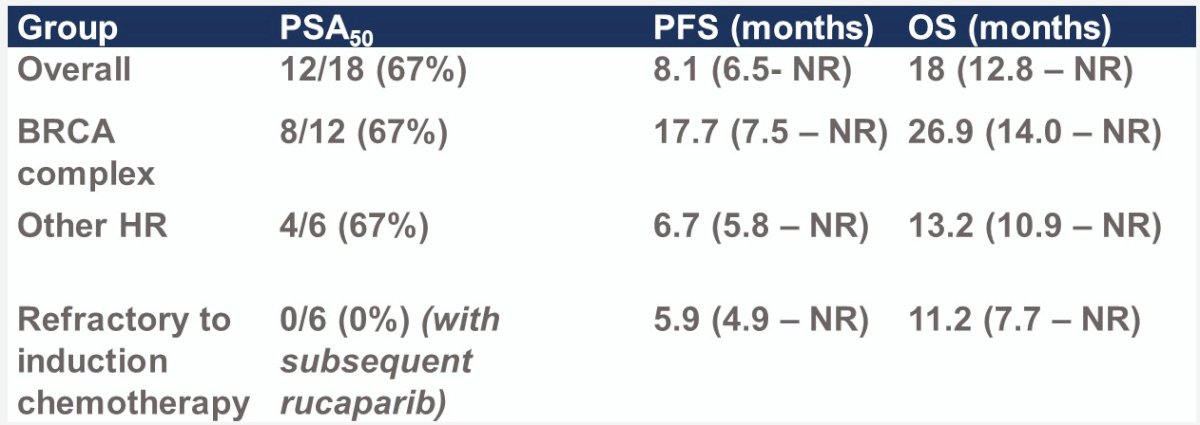
The median PFS was 8.1 months – the primary outcome was not met in the intent to treat population.

Patients with BRCA mutations had superior PFS compared to patients without such mutations (median: 17.7 versus 6.7 months):
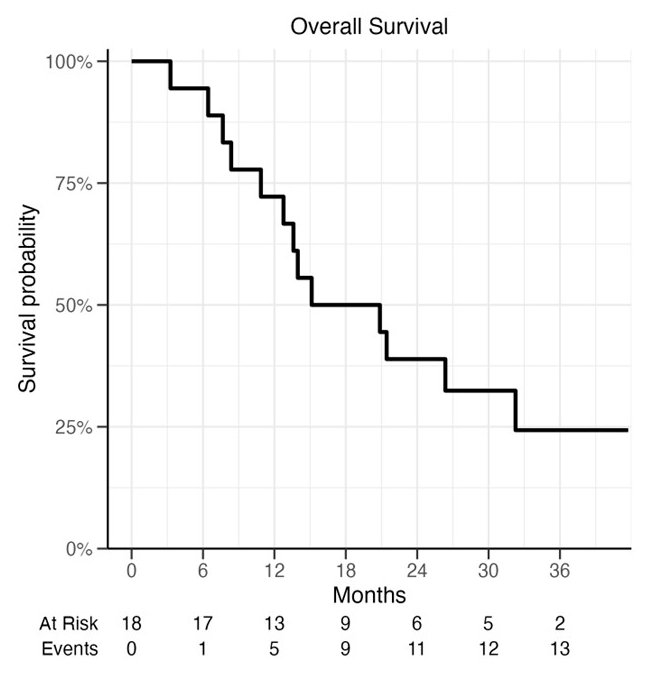
The median overall survival was 18 months.
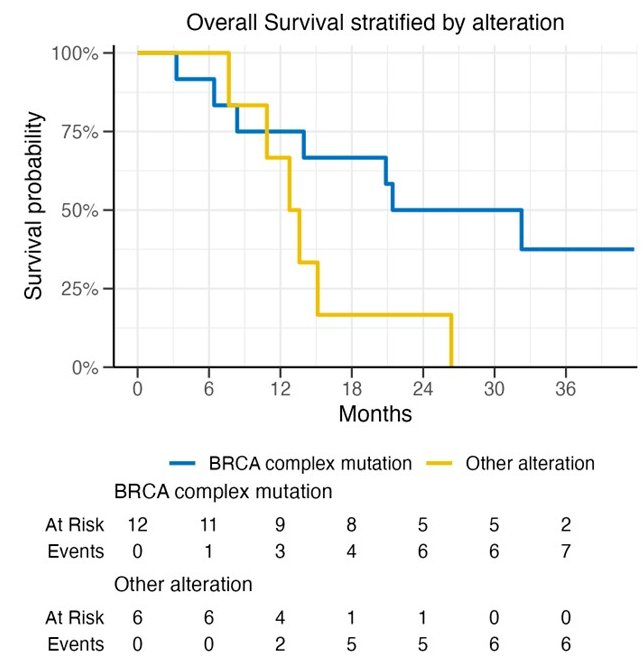
Similar to PFS, OS outcomes favored patients with BRCA mutations (median 26.9 months versus 13.2 months):
A PSA50 response was observed in 67% of patients (67% in both BRCA and non-BRCA mutated patients). None of the six patients refractory to induction chemotherapy subsequently achieved a PSA50 response with subsequent rucaparib in the maintenance phase.
Dr. Raychaudhuri concluded as follows:
- Platinum chemotherapy followed by maintenance rucaparib did not significantly increase PFS in patients with all HRR alterations compared to historical controls of PARPi monotherapy.
- Results were more encouraging in the BRCA complex group (17.7 months observed versus 18.2 months projected).
- Patients refractory to induction chemotherapy were not rescued by subsequent PARPi, suggesting overlapping mechanisms of resistance when a platinum-containing regimen is used prior to PARPi.
- The optimal use and sequencing of platinum with PARPi warrants further study in patients with HRR alterations (especially those in the BRCA complex) and is being investigated in the COBRA study (NCT04038502).
Presented by: Ruben Raychaudhuri, MD, Fellow, Division of Hematology Oncology, Department of Medicine, Fred Hutchinson Cancer Center, University of Washington, Seattle, WA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
References:
- Robinson D, Van Allen EM, Wu Y, et al. Integrative Clinical Genomics of Advanced Prostate Cancer. Cell. 2015;162(2): 454.
- Cheng HH, Prtichard CC, Boyd T, Nelson PS, Montgomery B. Biallelic Inactivation of BRCA2 in Platinum-sensitive Metastatic Castration-resistant Prostate Cancer. Eur Urol. 2016;69(6): 992-5.
- De Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020;382(22): 2091-102.
- Mateo J, Carreira S, Sandhu S, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N Engl J Med. 2015;373(18): 1697-708.


