(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31 and June 4 was host to the Rapid Oral Abstract Session: Genitourinary Cancer - Prostate, Testicular, and Penile. Dr. Yu-Wei Chen discussed the clinical utility of transcriptomic signatures to identify androgen receptor and neuroendocrine signaling in prostate cancer
Most prostate tumors exhibit adenocarcinoma histology. However, a small subset of castration-resistant prostate adenocarcinoma undergoes lineage transition with morphological transformation to small-cell/neuroendocrine prostate cancer (NEPC), furthermore, prostate adenocarcinoma can be mixed with NEPC with various degrees of small-cell carcinoma/neuroendocrine features. NEPC represents a particularly aggressive variant of prostate cancer, characterized by its relative independence from the androgen receptor (AR) signaling pathway which confers it a degree of resistance to conventional androgen deprivation therapy (ADT) and other standard treatments targeting the AR pathway.
Molecular characteristics of NEPC include a higher frequency of RB1 or TP53 gene alterations, which may involve concurrent loss of TP53/RB1. Previously, RNAseq-based androgen receptor (AR) signaling and NEPC signatures have been proposed and integrated into a NEPC score based on TP53/RB1 status. The aim of this study was to characterize the AR signaling and the NEPC signature across all prostate histology and site of metastases.
The investigators used previously validated AR signaling and Neuroendocrine transcriptomic signatures of prostate cancerto characterize the molecular features of three histologic subtypes:1
- Adenocarcinoma
- Neuroendocrine prostate cancer
- Mixed Adenocarcinoma-Neuroendocrine
Whole exome (WES) and whole transcriptome sequencing (WTS) were performed on prostate cancer tumors. They tested for DNA mutations and gene expression profiles as shown in the table below.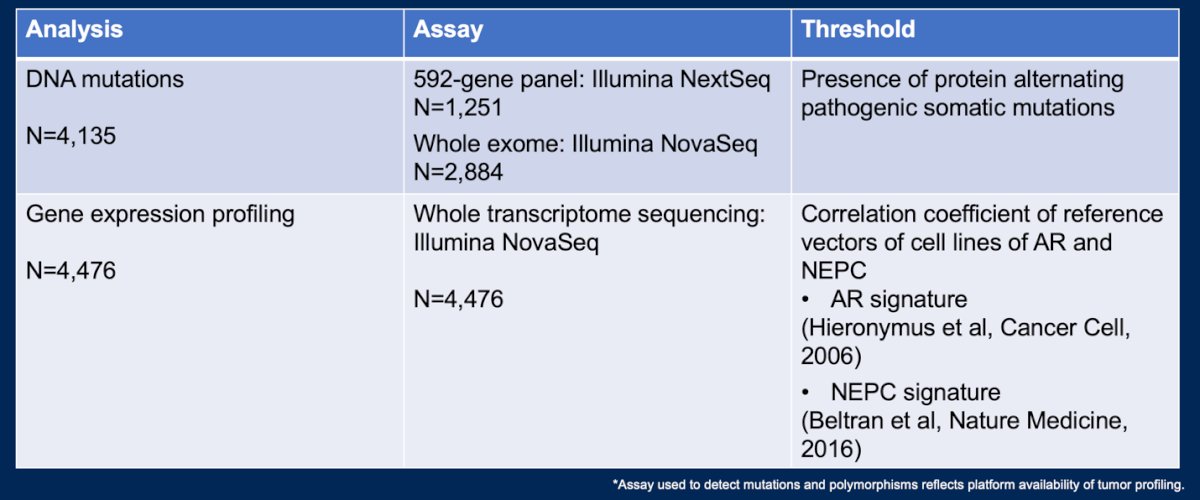
Different molecular subsets were defined by
- AR signaling signature: [AR score positive (+) vs negative (-)]
- Neuroendocrine prostate cancer gene signature: [NE score positive (+) vs negative (-)]
The investigators analyzed the interplay between AR signaling and NEPC in the previously defined histology patterns.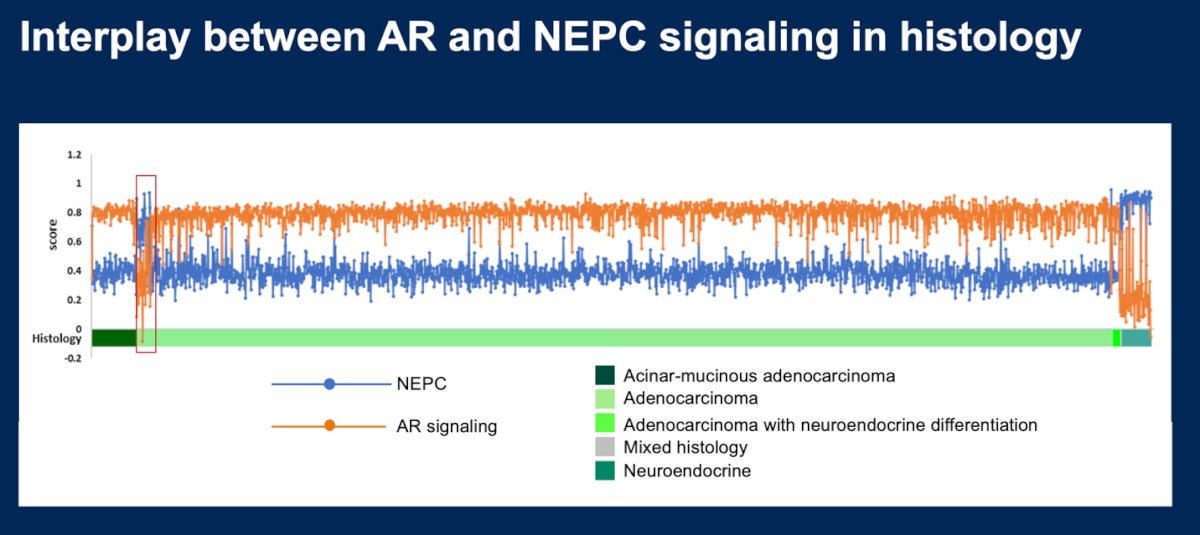
Moreover, the study investigated the prevalence of key molecular alterations including RB1, TP53, PTEN, AR, FANC, and SLFN11 expression. Additionally, genes encoding cell surface antigens with potential therapeutic implications were described across signature groups. Overall survival (OS) data were obtained from claims data and analyzed using Kaplan-Meier estimates.
Dr. Chen reported that 4,476 prostate tumors were analyzed in this study including 3,623 prostate adenocarcinoma, 56 neuroendocrine prostate cancers, and 25 tumors with mixed adenocarcinoma-neuroendocrine histology. Tissue from 2,641 (67%) tumors was collected from the prostate and 1,326 (33%) was collected from metastatic sites.
The distribution of the four AR/NEPC signature-defined subtypes were:
- AR+/NE+ (N=649, 14%)
- AR+/NE- (N=1614, 36%)
- AR-/NE+ (N=875, 20%)
- AR-/NE- (N=1358, 30%)
The baseline characteristics including histology and site of tissue collection are outlined in the table below:
The signature-defined subtypes were predominantly identified in:
- AR+/NE-: Prostate tissue (39%), lymph nodes (42%), and lung (35%)
- AR+/NE+: Prostate tissue (17%), lymph nodes (10%), and lung (16%)
- AR-/NE+: Bone (34%), liver metastasis (43%).
- AR-/NE-: Central nervous system (CNS) metastasis.
Most NE+ signature subtype was identified in bone and liver metastasis tissue.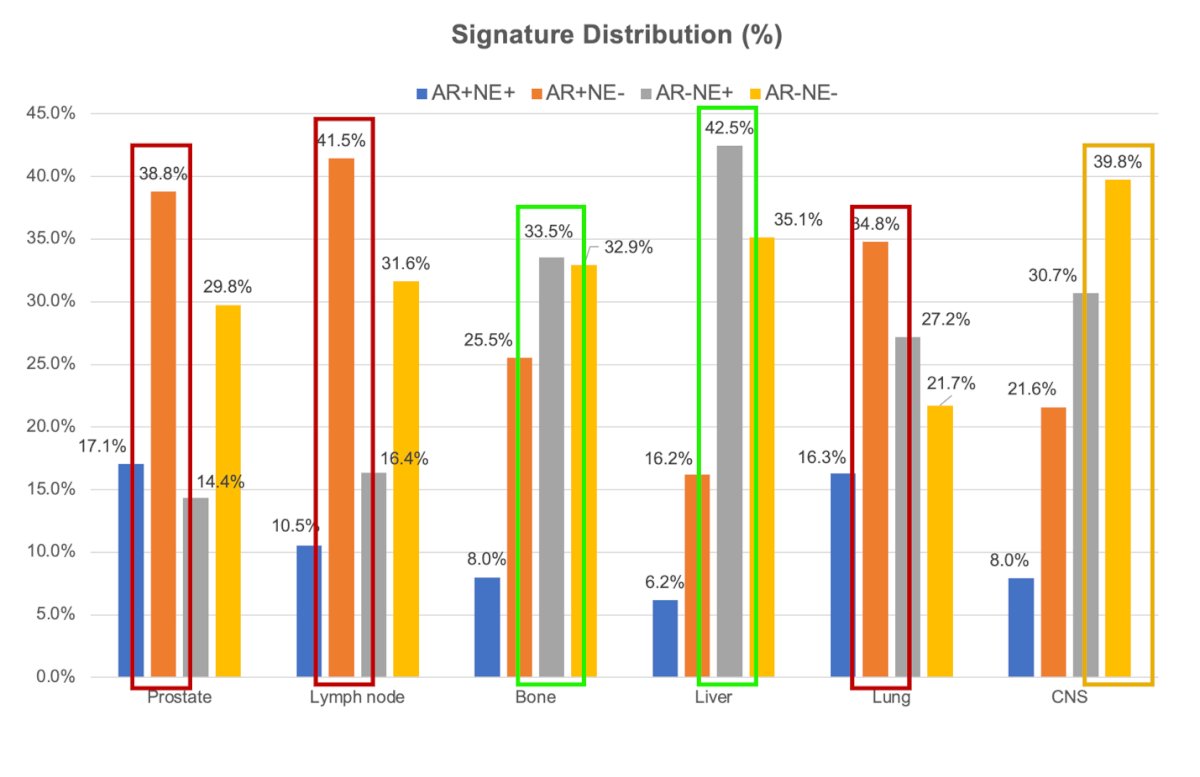
The most common gene alterations in each molecular subtype are outlined in the figure below. Briefly, AR-/NE+ had the highest expression of TP53 (46%) and RB1 (16.4%).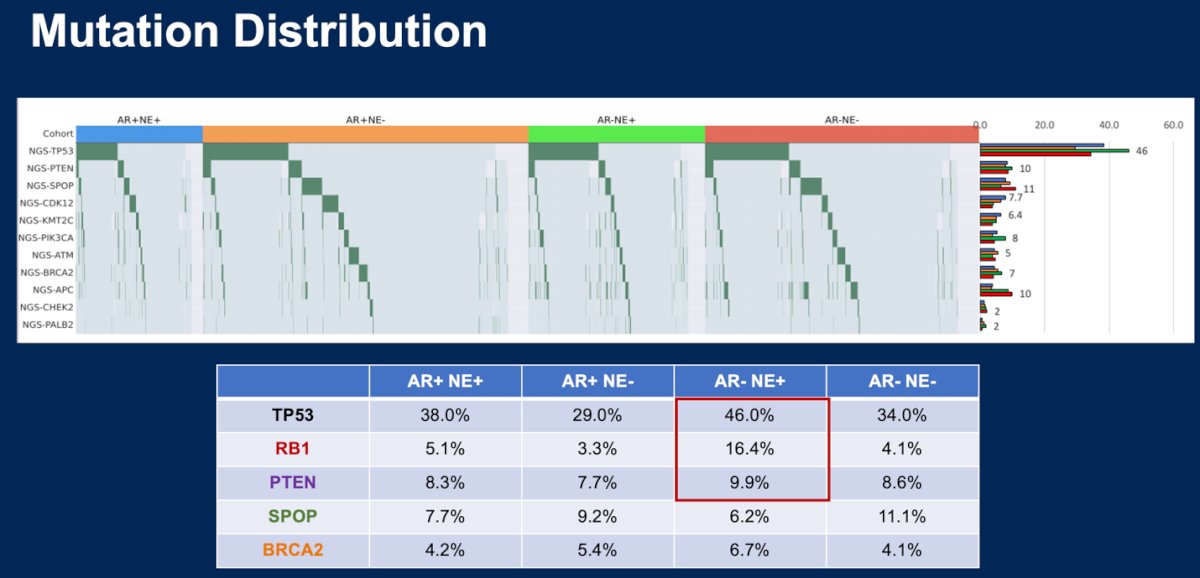
AR-/NE+ tumors had the lowest mRNA expression of FOLH1(PSMA), STEAP1, TACSTD2 (Trop-2), CD276 (B7-H3), and PSCA among the four groups. DLL3 expression was higher among NE+ subtypes, particularly AR-/NE+. FOLH1 and PSCA expression were higher among NE- subtypes. The complete expression profiles of each signature subtype are outlined below:
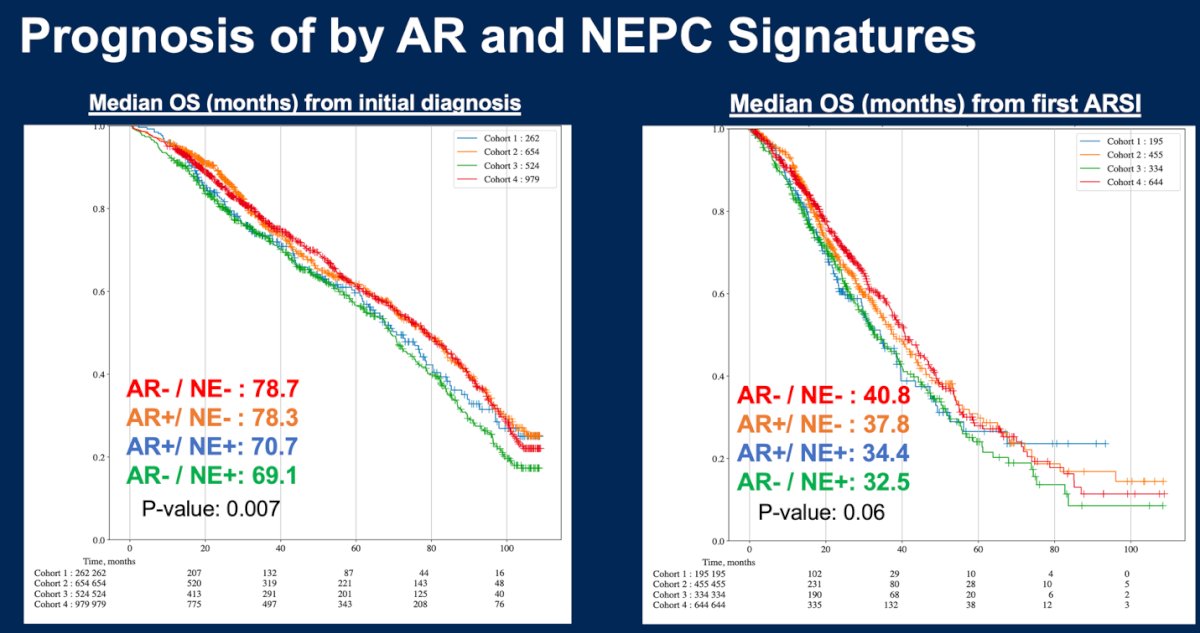
The median OS from initial diagnosis and first AR signaling inhibitor (ARSI) was longer in NE- than NE+ subtypes and this was significant for OS from initial diagnosis (p=0.007):
- AR+/NE-: 78.3 months
- AR-/NE- 78.7 months
- AR+/NE+ 70.7 months
- AR-/NE+ 69.1 months
To wrap up the presentation Dr. Chen discussed briefly the PREDICT trial (Alliance A032102). This trial is a phase II biomarker-driven study, that is going to use the signature-defined subtypes reported in this presentation to allocate treatment regimens in metastatic castration-resistant prostate cancer.
The following were the key takeaways of Dr Chen’s presentation:
- Molecularly distinct subtypes of prostate cancer can be further characterized by AR signaling signature, NE signature, and co-occurring molecular alterations gene expression signature
- Gene signatures and molecular subgroups differ between metastatic sites (lymph nodes, bone, liver, lung, CNS) when compared to prostate tumors
- NE signature (+) is associated with worse overall survival
- Understanding the molecular subtypes of prostate cancer will help inform personalized treatment strategies in advanced prostate cancer
Presented by: Yu-Wei Chen, MD, MS, Medical Oncologist, Moores Cancer Center, UC San Diego Health, San Diego, California
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31st and June 4th.
Related content: Prostate Cancer Diversity Revealed: AR and Neuroendocrine Signatures - Yu-Wei Chen
References


