(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31 and June 4 was host to the Rapid Oral Abstract Session: Genitourinary Cancer - Prostate, Testicular, and Penile. Dr. Luca Faustino Valle presented their retrospective cohort study evaluating oncogenic alteration rates, race, and prostate cancer specific mortality in veterans with metastatic prostate cancer undergoing somatic tumor next generation sequencing testing.
It's undeniable that Black men in the U.S. exhibit higher prostate cancer mortality rates and higher incidence of metastasis at diagnosis than White men at the population level. Data from Surveillance, Epidemiology, and End Results (SEER) showed that 1 in 7 Black men will be diagnosed with prostate cancer in his lifetime versus 1 in 9 White men: 1.6-fold increase in age-adjusted prostate cancer incidence among Black (1). However, data from systems with equal access to healthcare such as the Veterans Affairs (VA) system suggest that Black men with prostate cancer may exhibit similar survival compared to White men (2), which represents an ideal healthcare scenario for the analysis of oncogenic alteration rates, race, and prostate cancer specific mortality.
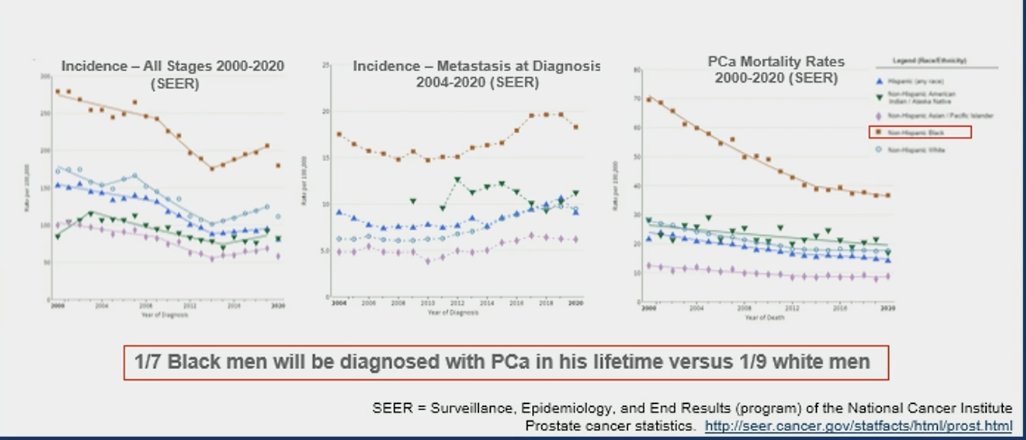
Numerous clinical practice guidelines advocate for the implementation of next-generation sequencing (NGS) in the assessment of tumors from patients diagnosed with metastatic prostate cancer (PCa). This approach aims to discover alterations that could guide treatment decisions and improve patient outcomes. Dr. Valle mentioned that for this study, the investigators sought to determine associations between alteration frequencies of PCa-related genes and pathways, self-identified race, and PCa-specific mortality in Veterans using the VA National Precision Oncology Program cohort.
This was a retrospective cohort study evaluating the association of somatic mutations with race in patients with metastatic PCa in the equal access VA healthcare system who underwent NGS. The NGS data was linked to clinicopathologic and social determinants of health data elements. The investigators used multivariate logistic regression to explore the associations between race and genomic alteration frequencies, adjusting for the specimen tested and clinical and social determinants of health co-variates. The association between race, somatic mutation frequencies, and prostate cancer mortality was explored using Cox proportional hazards models.
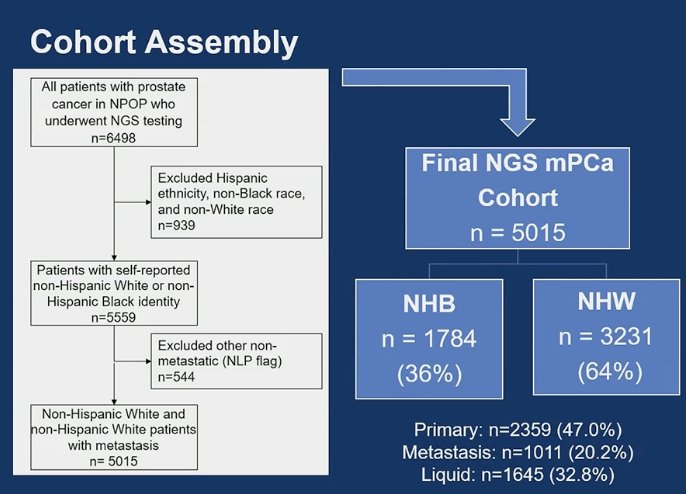
The investigators included 5,015 patients with metastatic prostate cancer in the analysis. Within this cohort, individuals identified themselves as:
- Non-Hispanic Black (NHB): 1,784 patients (36%)
- Non-Hispanic White (NHW): 3,231 patients (64%)
NGS was performed on the primary tumor tissue of 2,359, metastasis in 1,011, and circulating-free DNA (cfDNA) in 1,644. The study design is depicted in the figure below.
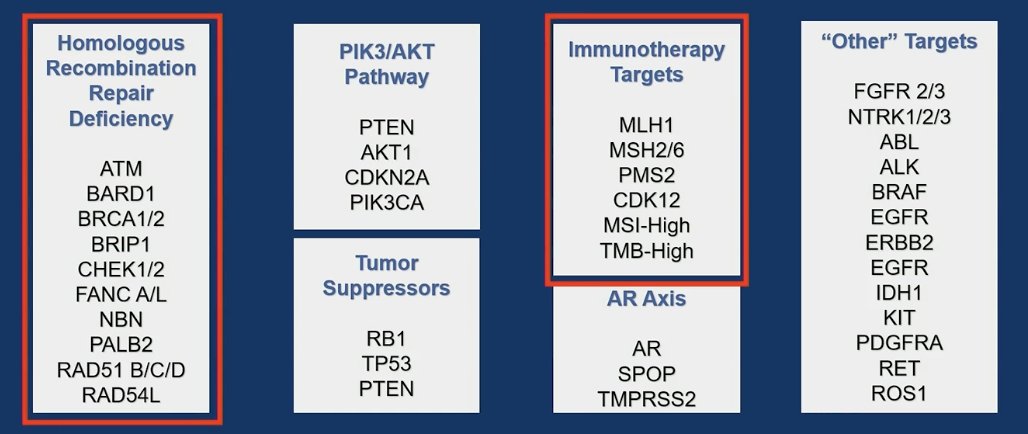
The full list of genes included in the study encompasses the homologous recombination repair (HRR) pathway genes, tumor suppressor genes, the PI3K/AKT pathway, the Androgen receptor (AR) axis, immunotherapy targets, and other relevant targets. A detailed depiction of these genes is provided below, with boxes encircled in red indicating the targetable genes.
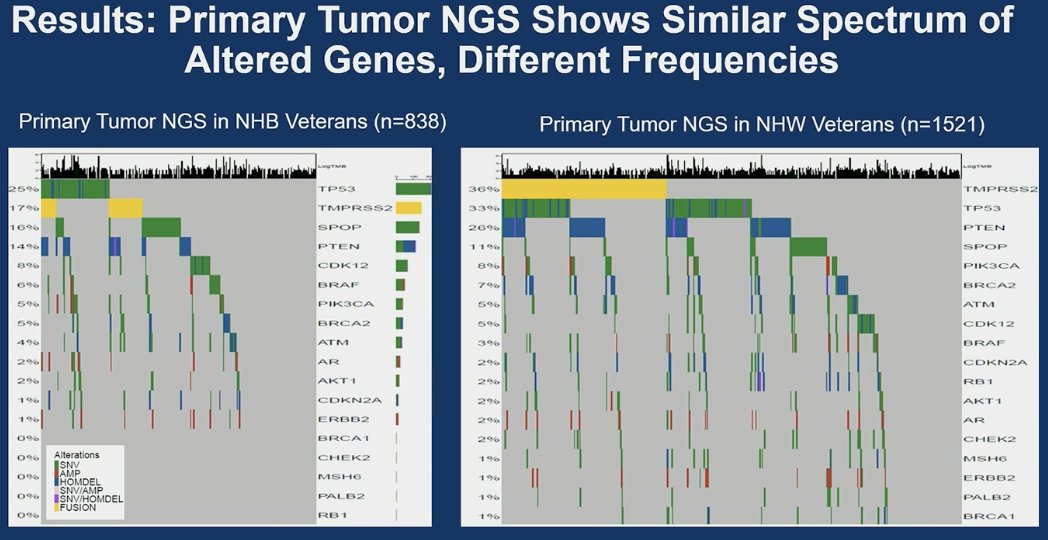
Overall, the NHB Veterans were younger, had higher PSA at PCa diagnoses, were less likely to report agent Orange (chlorophenoxy herbicides 2,4-D and 2,4,5-T ) exposure, and resided in more deprived neighborhoods (ADI).
Primary tumor NGS revealed a comparable spectrum of altered genes in NHB and NHW veterans, albeit at distinct frequencies. TP53 alterations were the most prevalent in NHB individuals (25%), followed by TMPRSS2 (17%) and SPOP (16%). In NHW individuals, the most frequent gene alterations, in descending order, were TMPRSS2 (36%), TP53 (33%), PTEN (22%), and SPOP (11%).
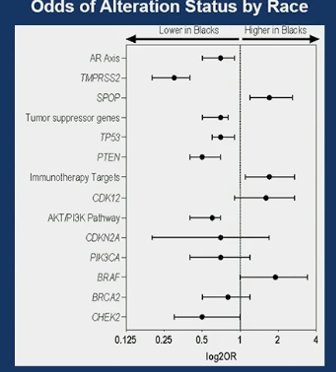
In univariate/unadjusted analysis NHB race/ethnicity was significantly associated with lower rates of genomic alterations in:
- The AKT/PI3K pathway (p<0.001)
- AR axis (p<0.001)
- Tumor suppressor genes (TP53and PTEN) (p<0.001)
After adjusting for the type of tissue sequenced (prostate, metastasis or cfDNA) and clinicopathologic variables they found that NHB race/ethnicity was significantly associated with a higher rate of genomic alterations in immunotherapy targets such as SPOP and BRAF(p<0.05).
In multivariate analysis, adjusting for several factors including: NGS analyte, age at PCa diagnosis, PSA at PCa diagnosis, Grade group, age at first NGS testing, histology at first NGS testing, de novo metastatic disease at NGS testing, castrate-resistant status at NGS testing, military exposure, Charlson Comorbidity Index, Area Deprivation Index (ADI), and smoking status. Immunotherapy targets and SPOP alterations were significantly more prevalent in Black individuals.
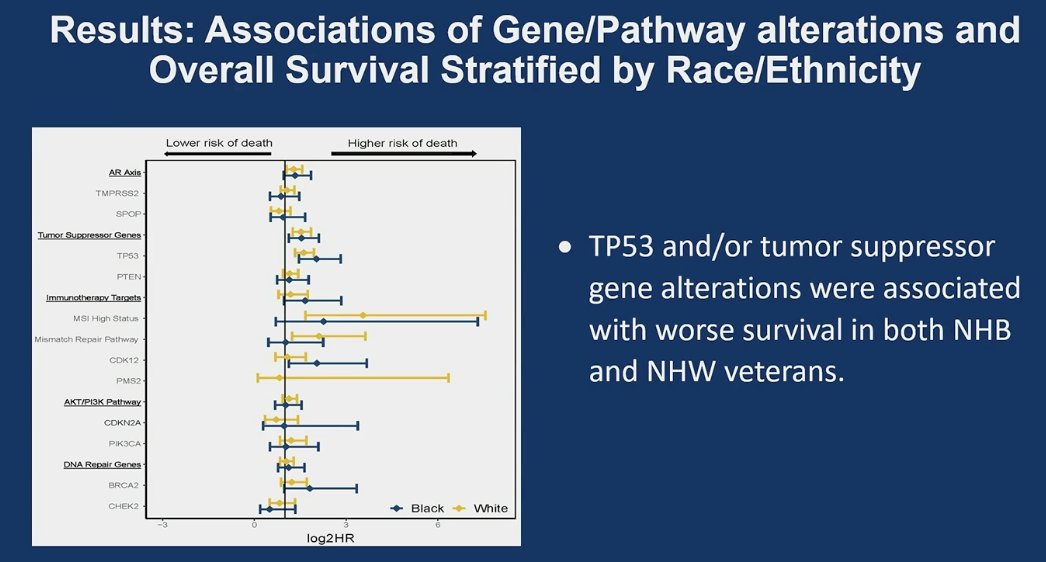
Alterations in TP53 (HR 1.5, 95% CI 1.1-2.0, p=0.007), and tumor suppressor pathways (RB1, PTEN) (HR 1.3 95% CI, 1.0-1.8, p=0.04) conferred a significantly higher risk of prostate cancer mortality in all men on adjusted analysis, race was not independently associated with prostate cancer mortality.
Dr Valle concluded his presentation with the following key points:
- In this retrospective cohort significant differences in alteration rates of several oncogenic genes/pathways were identified based on patient race/ethnicity.
- NHB patients were more likely to have genomic alterations in SPOP and BRAF, however, there were lower rates of genomic alterations in the AKT/PI3K pathway, the AR axis and tumor suppressor genes.
- In multivariable analysis NHB men had significantly higher frequencies of alterations in immunotherapy targets (including MSI high status) than NHW men.
- Alterations in tumor suppressor genes were significantly associated with Prostate cancer mortality. However, race/ethnicity was not.
- Next generation sequencing identifies candidates for precision oncology treatments, which could contribute to equitable outcomes in patients with metastatic prostate cancer.
Presented by: Luca Faustino Valle, MD, Assistant Professor in the Department of Radiation Oncology at the David Geffen School of Medicine at the University of California, Los Angeles
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
References:
- Chowdhury-Paulino IM, Ericsson C, Vince R Jr, Spratt DE, George DJ, Mucci LA. Racial disparities in prostate cancer among black men: epidemiology and outcomes. Prostate Cancer Prostatic Dis. 2022 Sep;25(3):397-402. doi: 10.1038/s41391-021-00451-z. Epub 2021 Sep 2. PMID: 34475523; PMCID: PMC8888766.
- Dess RT, Hartman HE, Mahal BA, Soni PD, Jackson WC, Cooperberg MR, Amling CL, Aronson WJ, Kane CJ, Terris MK, Zumsteg ZS, Butler S, Osborne JR, Morgan TM, Mehra R, Salami SS, Kishan AU, Wang C, Schaeffer EM, Roach M 3rd, Pisansky TM, Shipley WU, Freedland SJ, Sandler HM, Halabi S, Feng FY, Dignam JJ, Nguyen PL, Schipper MJ, Spratt DE. Association of Black Race With Prostate Cancer-Specific and Other-Cause Mortality. JAMA Oncol. 2019 Jul 1;5(7):975-983. doi: 10.1001/jamaoncol.2019.0826. PMID: 31120534; PMCID: PMC6547116.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Prostate Cancer. Version 3.2024. Accessed [June 3, 2024]. Available from: [https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf]


