(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between May 31 and June 4 was host to the Rapid Oral Abstract Session: Genitourinary Cancer - Prostate, Testicular, and Penile. Dr. Mark Linch presented results from the NEPTUNES (NCT03061539), a multi-centre two-cohort, biomarker-selected phase 2 trial evaluating Nivolumab (anti-PD1) and ipilimumab (anti-CTLA4) for metastatic prostate cancer with an immunogenic signature.
Dr. Linch initiated his presentation by addressing the limited responses observed with immune checkpoint inhibitor monotherapy in patients with metastatic castration-resistant prostate cancer (mCRPC) (1). This phenomenon is largely attributed to the prevalence of a "cold" tumor immune microenvironment. Anti-CTLA4 therapy has been found to induce T-cell infiltration in prostate cancer, but most importantly, the combination of anti-CTLA4 and anti-PD1 has demonstrated a signal of activity in unselected patients with mCRPC in the preliminary analysis of the CheckMate 650 Trial combining Nivolumab+ipilimumab (2). Moreover, up to 20% of patients with high-risk PCa have high tumor infiltrating lymphocytes (TILs), which have been shown, albeit inconsistently, to be prognostic in PCa. (3)
The investigators hypothesized that patients with mCRPC exhibiting a positive immunogenic signature (ImS+) in the NEPTUNES trial would be more likely to respond to a combination of anti-PD1 and anti-CTLA4. This is the first report of Cohort 2 and an updated report of Cohort 1 for men with mCRPC who are ImS+ treated with nivolumab and ipilimumab in the NEPTUNES study.
This study compared two different dose schedules for Nivolumab + Ipilimumab in patients with ImS+ mCRPC. For this trial patients with mCRPC who progressed following at least one line of therapy and ImS+ were eligible. For the study ImS+ was defined as having ≥1 of the following criteria:
- Mismatch repair deficient (MMRD) by immunohistochemistry (IHC)
- DNA damage repair deficient (DDRD) detected by the UW-OncoPlex targeted exome sequencing assay
- High Tumour infiltrating lymphocytes (TILs) on multiplexed IHC (≥20% of nucleated cells)
The NEPTUNES trial involved two patient cohorts with different doses of Nivolumab and Ipilimumab and dose schedules:
Cohort 1:- Nivolumab 1 mg/kg + ipilimumab 3 mg/kg every three weeks for a maximum of 4 doses
- 6-week gap after last combination dose
- 480 mg flat dose of nivolumab every 4 weeks for up to one year, or until progression, unacceptable toxicity or withdrawal of consent.
- Nivolumab 3 mg/kg + ipilimumab 1 mg/kg every three weeks for a maximum of 4 doses
- 3-week gap after last combination dose
- 480 mg flat dose of nivolumab every 4 weeks for up to one year, or until progression, unacceptable toxicity or withdrawal of consent.
Patients were required to have ongoing androgen deprivation therapy (ADT) to maintain serum testosterone < 1.73 nmol/L. The study design is outlined below:
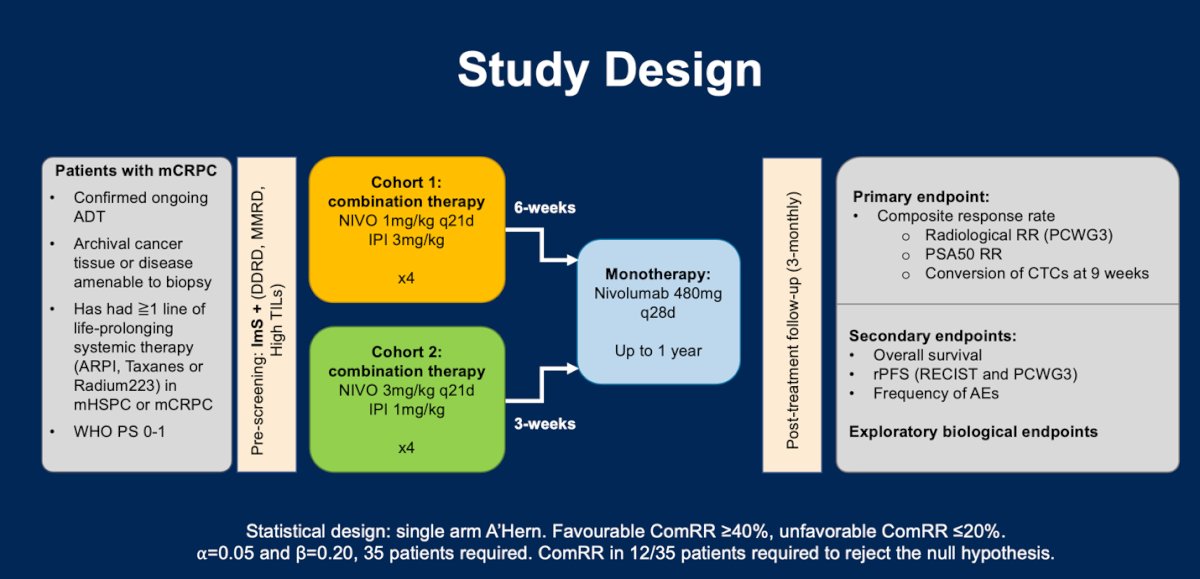
The primary endpoint of this trial was a composite endpoint defined as composite response rate (CRR), per protocol a CRR ≥40% would be clinically favourable, and a CRR of ≤20% was considered an unfavorable CRR (n=35 patients, ⍺=0.05 and β=0.20). a CRR was defined as achieving ≥1 of the following milestones:
- Confirmed radiological response by RECIST 1.1
- Confirmed PSA response ≥50%
- Conversion of circulating tumour cells (CTC) at week 9.
Secondary endpoints included toxicity (frequency of adverse events), overall survival (OS) and radiological progression free survival (rPFS) per RECIST and PCWG3.
Between May 2018 and June 2022, the investigators identified 119/380 (31%) screened patients with ImS+. Of the ImS+ patients 69 (29%) were enrolled in Cohort 1 and 50 (34%) were enrolled in Cohort 2.

The baseline patient characteristics of Cohorts 1 and 2 are depicted in the table below. Notably, 89% in Cohort 1 and 71% in Cohort 2 have received previous treatment with Docetaxel, and most patients received more than one line of therapy.

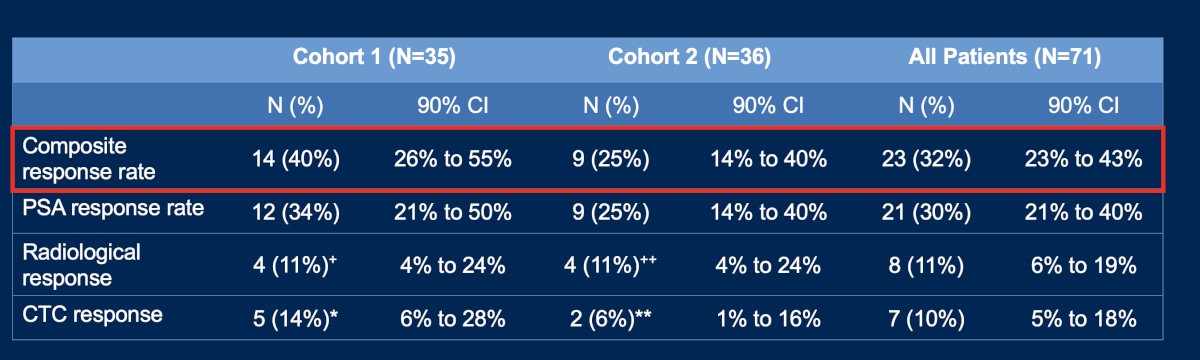
The CRR in Cohort 1 was 40%, and in Cohort 2, it was 25%. The combined CRR in both cohorts was 23/71 (32%). Dr. Linch noted that the median duration of response was longer in Cohort 1 (10.4 months versus 6.4 months) than in Cohort 2. The table below breaks down response according to composite response, PSA response, radiological (RECIST) response, and CTC response.
After a median follow-up of 47 months for Cohort 1 and 21 months for Cohort 2, the median OS was 16.2 (95% CI 9.2-22.8) months and 15.2 (95% CI 8.9-NR) months, respectively.

The Swimmers plot combining Cohorts 1 and 2 showed the duration of response intervals in months for radiological, PSA, and CTC responses, along with the CRR in these patients.
The ImS+ determinants in responding patients were:
- MMRD: 8/10 (80%)
- BRCA1/2: 4/8 (50%)
- high TILs: 8/21 (33%)
- CDK12: 2/8 (25%)
- ATM: 1/13 (8%)
- CHD1: 1/9 (11%)

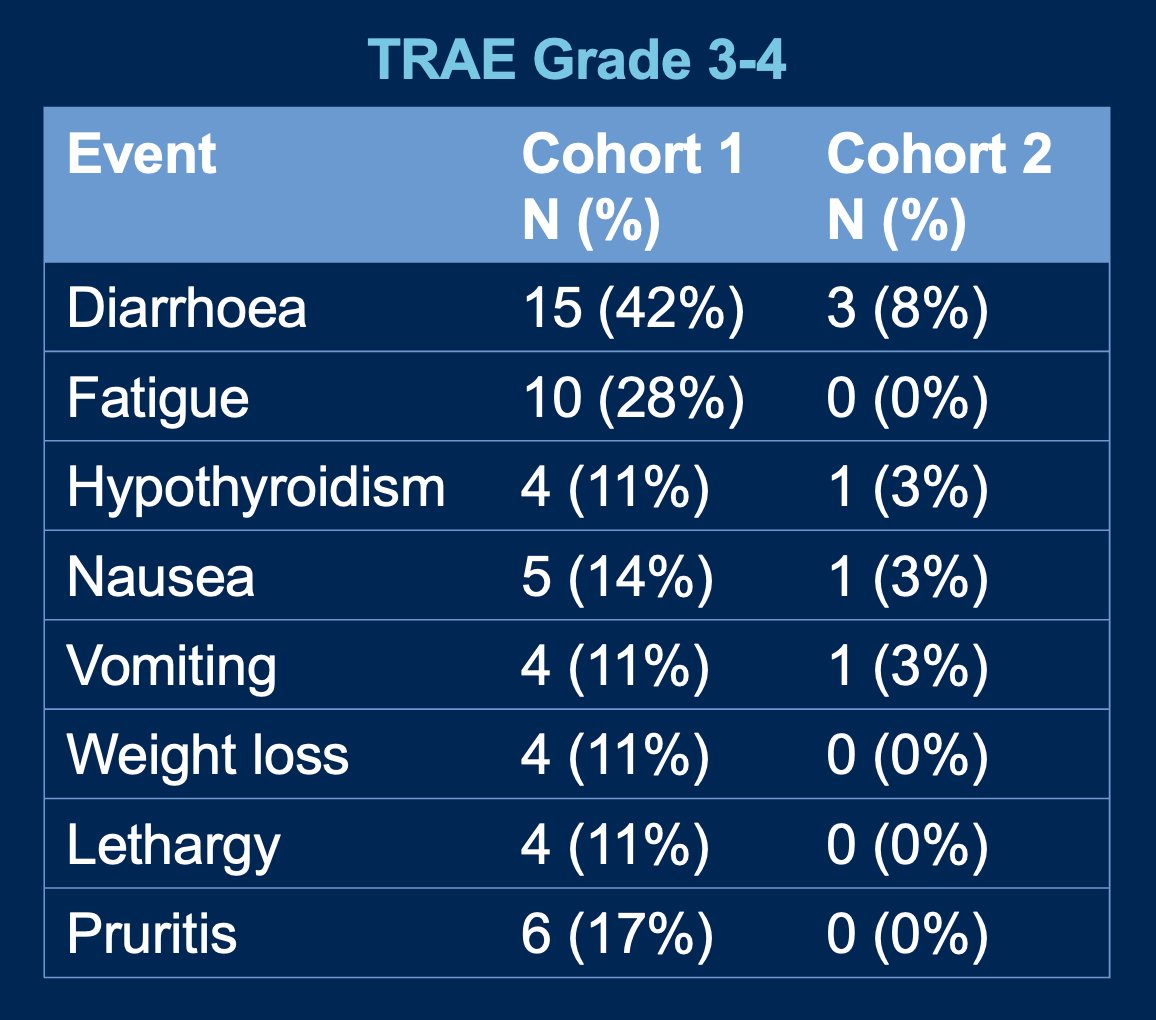
Grade ≥ 3 treatment-related adverse events (TRAEs) occurred in 22/35 (63%) in Cohort 1 and 11/36 (31%) in Cohort 2. The most common Grade ≥ 3 TRAEs was diarrhoea present in 15 (42%) and 3 (8%) patients for Cohort 1 and 2, respectively. Other Grade ≥ 3 TRAEs are shown in the graphic below.
Dr. Linch summarized their findings with the following key messages:
- Combination treatment with Nivolumab+Ipilimumab demonstrated anti-tumour activity in ImS+ patients with mCRPC (CRR 40% in Cohort 1 and 25% in Cohort 2).
- Overall survival was similar between both dosing schedules (16 vs. 15 months).
- Responses were enriched in patients with MMRD, BRCA1/2, and TILs.
- Cohort 2 treatment schedule (NIVO 3 + IPI 1) was better tolerated.
- Further study (phase 3 trial) of Nivolumab+Ipilimumab in biomarker-selected mCRPC patients is warranted.
Presented by: Mark David Linch, MD, PhD, MBChB, Medical Oncologist at the University College of London Cancer Institute.
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024
References
- Antonarakis ES, Piulats JM, Gross-Goupil M, Goh J, Ojamaa K, Hoimes CJ, Vaishampayan U, Berger R, Sezer A, Alanko T, de Wit R, Li C, Omlin A, Procopio G, Fukasawa S, Tabata KI, Park SH, Feyerabend S, Drake CG, Wu H, Qiu P, Kim J, Poehlein C, de Bono JS. Pembrolizumab for Treatment-Refractory Metastatic Castration-Resistant Prostate Cancer: Multicohort, Open-Label Phase II KEYNOTE-199 Study. J Clin Oncol. 2020 Feb 10;38(5):395-405. doi: 10.1200/JCO.19.01638. Epub 2019 Nov 27. PMID: 31774688; PMCID: PMC7186583.
- Sharma P, Pachynski RK, Narayan V, Fléchon A, Gravis G, Galsky MD, Mahammedi H, Patnaik A, Subudhi SK, Ciprotti M, Simsek B, Saci A, Hu Y, Han GC, Fizazi K. Nivolumab Plus Ipilimumab for Metastatic Castration-Resistant Prostate Cancer: Preliminary Analysis of Patients in the CheckMate 650 Trial. Cancer Cell. 2020 Oct 12;38(4):489-499.e3. doi: 10.1016/j.ccell.2020.08.007. Epub 2020 Sep 10. PMID: 32916128.
- Linch M, Goh G, Hiley C, Shanmugabavan Y, McGranahan N, Rowan A, Wong YNS, King H, Furness A, Freeman A, Linares J, Akarca A, Herrero J, Rosenthal R, Harder N, Schmidt G, Wilson GA, Birkbak NJ, Mitter R, Dentro S, Cathcart P, Arya M, Johnston E, Scott R, Hung M, Emberton M, Attard G, Szallasi Z, Punwani S, Quezada SA, Marafioti T, Gerlinger M, Ahmed HU, Swanton C. Intratumoural evolutionary landscape of high-risk prostate cancer: the PROGENY study of genomic and immune parameters. Ann Oncol. 2017 Oct 1;28(10):2472-2480. doi: 10.1093/annonc/mdx355. PMID: 28961847; PMCID: PMC5815564.


