(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured a session on prostate cancer, and a presentation by Dr. Daniel Petrylak discussing initial results of a phase 1/2 study of ARV-766 in metastatic castration-resistant prostate cancer (mCRPC). Patients with mCRPC inevitably develop resistance to available therapies (ie. novel hormonal agents) and experience disease progression.
Certain mutations that can develop in the ligand-binding domain (amino acids 671–920) of the AR gene during mCRPC treatment have been associated with poor outcomes. ARV-766 is a novel, potent, orally administered PROTAC androgen receptor degrader that targets wild-type AR and clinically relevant AR ligand-binding domain mutants, including the most prevalent AR L702H, H875Y, and T878A mutations: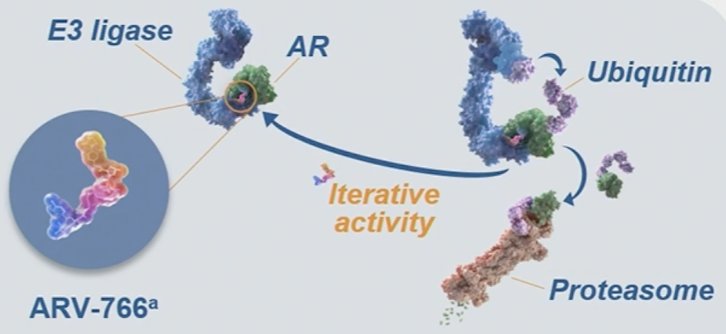
At the 2024 ASCO annual meeting, Dr. Petrylak and colleagues reported the initial results from a phase 1/2 study of ARV-766 in men with mCRPC and disease progression on prior novel hormonal agent therapy.
Eligible patients had progressive mCRPC and ongoing ADT. The phase dose-escalationon portion evaluated the safety and tolerability of escalating doses of ARV-766 (20–500 mg once daily) in patients who had progressed on ≥2 prior systemic therapies (including ≥1 novel hormonal agents). The phase 2 cohort expansion portion is evaluating the clinical activity and safety of 2 doses of ARV-766 (100 or 300 mg once daily) in patients who had received 1–3 prior novel hormonal agents and ≤2 prior chemotherapy regimens:
Safety in all patients treated with ARV-766 across the phase 1/2 study and clinical activity (proportion of patients with best PSA declines of ≥50% [PSA50] after ≥1 month of PSA follow-up) in the subgroup of patients with AR ligand-binding domain mutations was reported at ASCO 2024.
Dose-dependentt increases in ARV-766 exposure up to 320 mg daily were observed in phase 1: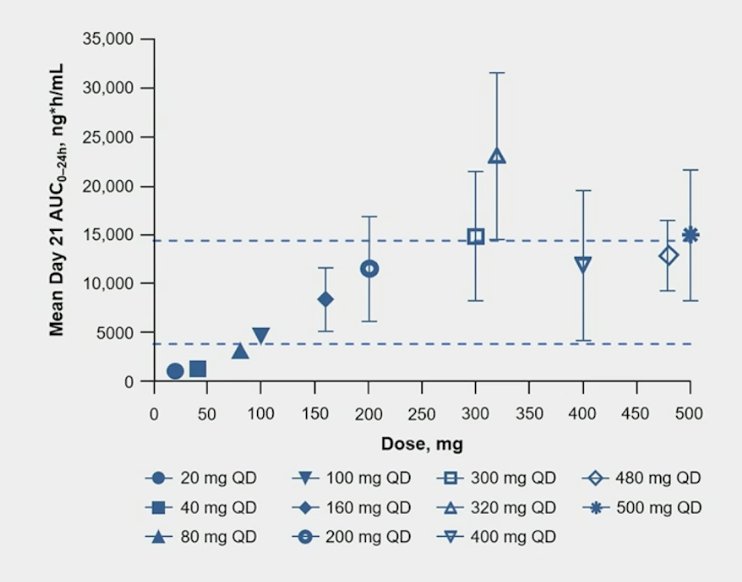
As of April 15, 2024, 123 patients received ARV-766, including 53 patients with Aligand-bindingng domain mutations. Patients had received a median of 4 prior therapies (range: 1–10), including 56% with ≥1 prior taxane and 46% with ≥2 prior novel hormonal agents. Patients with AR ligand-binding domain mutations had received a median of 4 prior therapies (range: 1–10), including 58% with ≥1 prior taxane and 55% with ≥2 prior novel hormonal agents: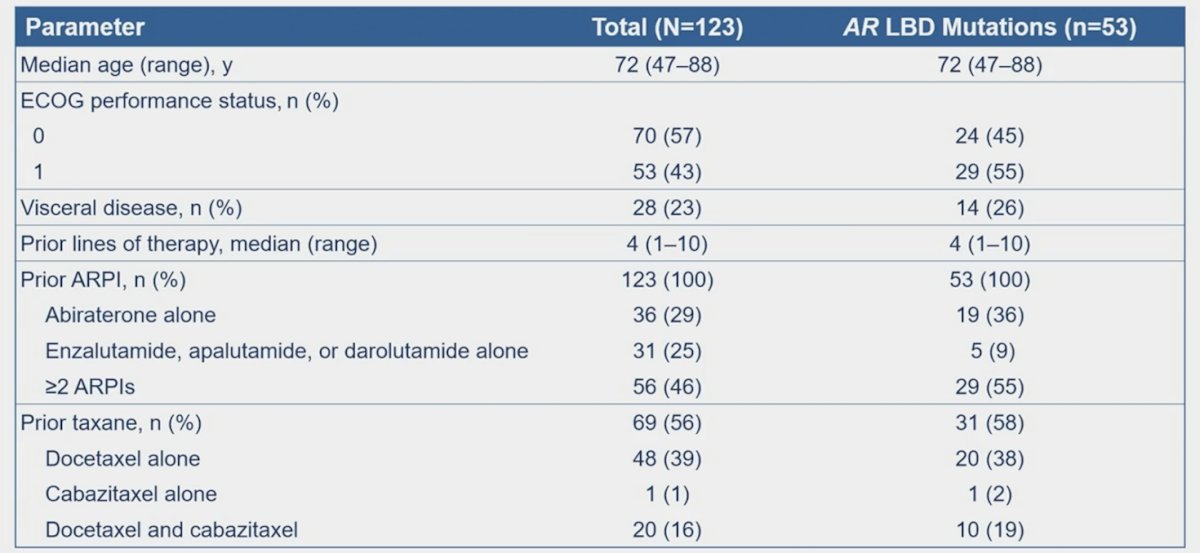
In phase 1, there were no dose-limiting toxicities, and a maximum tolerated dose was not reached. Across 123 phase 1/2 patients, any grade treatment-emergent adverse events occurred in 118 (96%) patients, including 46 (37%) grade 3/4treatment-emergent adverse events, and 3 (2%) grade 5treatment-emergent adverse events. Nine patients (7%) treatment-emergent adverse events that led to a dose reduction of ARV-766, and 10 (8%) treatment-emergent adverse events that led to discontinuations of ARV-766. Treatment-emergent adverse events in >=10% of patients of interest included 33% any grade fatigue, 20% any grade nausea, and 15% any grade diarrhea:
Among patients with AR ligand-binding domain mutations, PSA50 was 43% and PSA30 was 51%: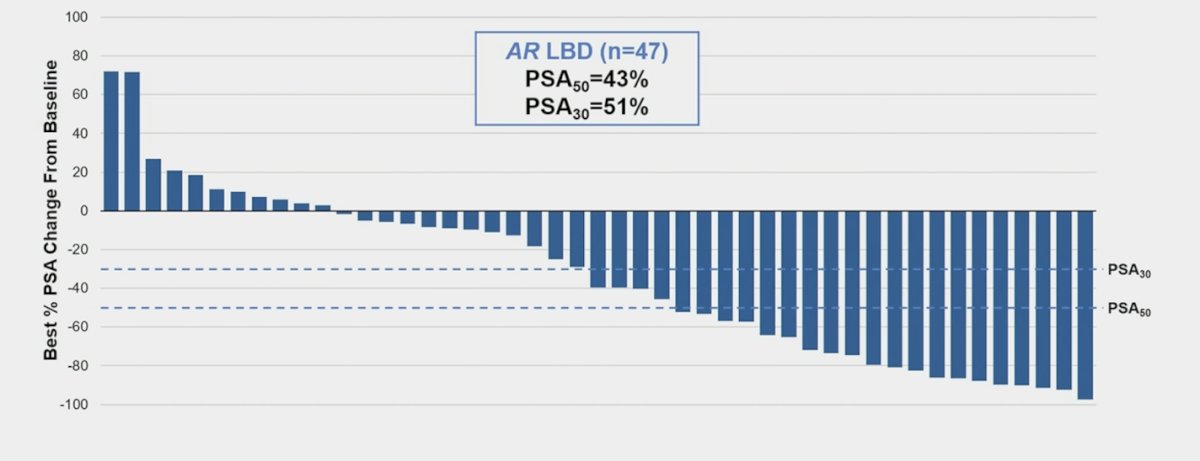
The unconfirmed objective response rate among AR ligand binding domain patients (n = 20) is 30% with several patients continuing therapy: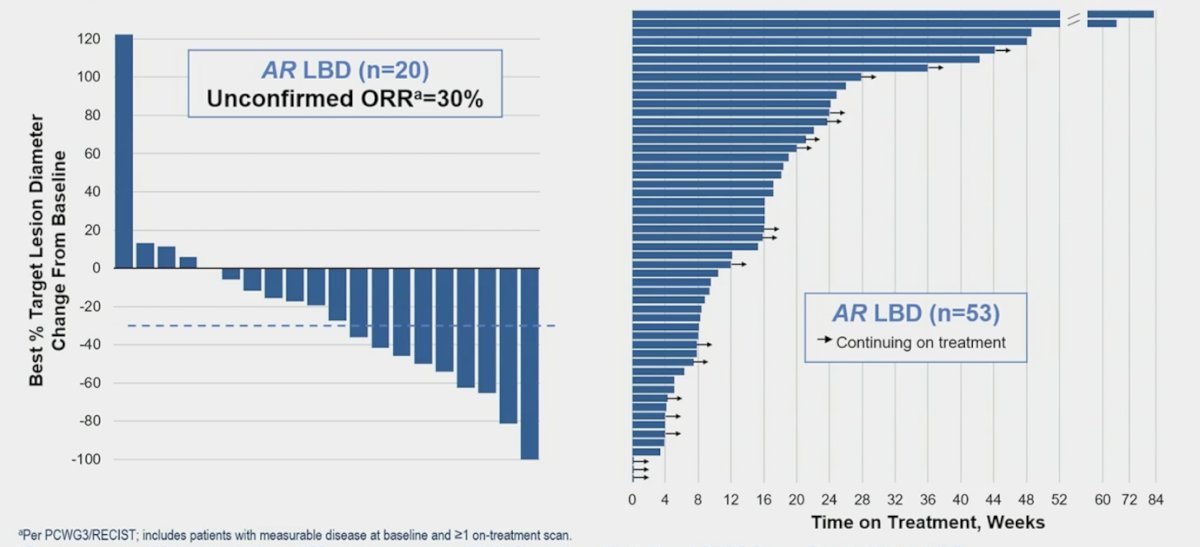
Dr. Petrylak concluded his presentation discussing initial results of a phase 1/2 study of ARV-766 in mCRPC with the following take home messages:
- In this phase 1/2 study of pretreated patients with mCRPC, ARV-766 was well tolerated and showed promising clinical activity in those with tumors harboring AR ligand-binding domain mutations (PSA50 of 43%)
- ARV-766 warrants further development in advanced prostate cancer
Presented by: Daniel P. Petrylak, MD, Oncologist, Smilow Cancer Center, Yale School of Medicine, New Haven, CT
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
Related content: ARV-766: Efficacy and Safety of a Second-Generation AR Degrader in Prostate Cancer - Daniel Petrylak


