(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago, IL between May 31st and June 4th, 2024 was host to a session addressing techniques to minimize the effects of androgen deprivation therapy (ADT) while explore alternatives in prostate cancer management. Dr. Ashwin Sachdeva discussed the appropriate use and timing of bone resorptive agents in patients with advanced prostate cancer.
Bone health is critical across the prostate cancer disease spectrum and is not limited to patients with metastatic disease. Patients with non-metastatic, hormone-sensitive prostate cancer may receive 6 months to 3 years of ADT concurrently with radiotherapy and this may lead to loss of bone mineral density. In patients with metastatic disease, another complicating factor is the increased bone turnover secondary to osseous metastases and skeletal complications/skeletal-related events. As such, the goals of bone health are prevention of osteoporotic fractures across the disease spectrum and, additionally, preventing/delaying skeletal-related events in those with M1 disease.

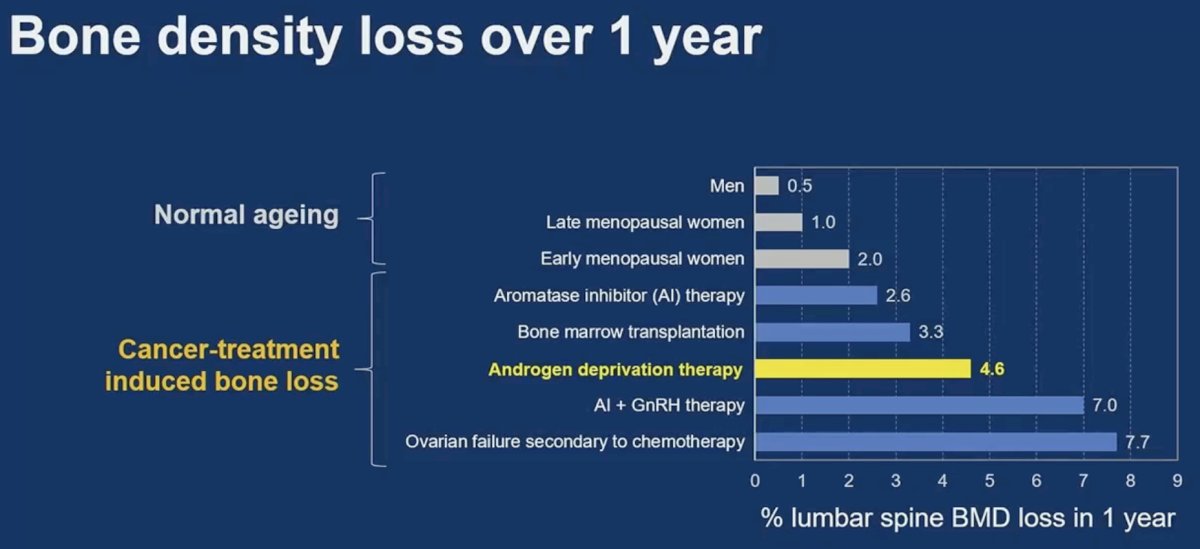
What is the true impact of ADT on bone density loss? The percentage of lumbar spine bone mineral density loss in one year is approximately 5% for men receiving ADT, compared to 0.5% for ‘normal ageing’ men.1 As such, we see an almost 10-fold increase in bone mineral density loss in these patients, highlighting the adverse effects of ADT on bone health.
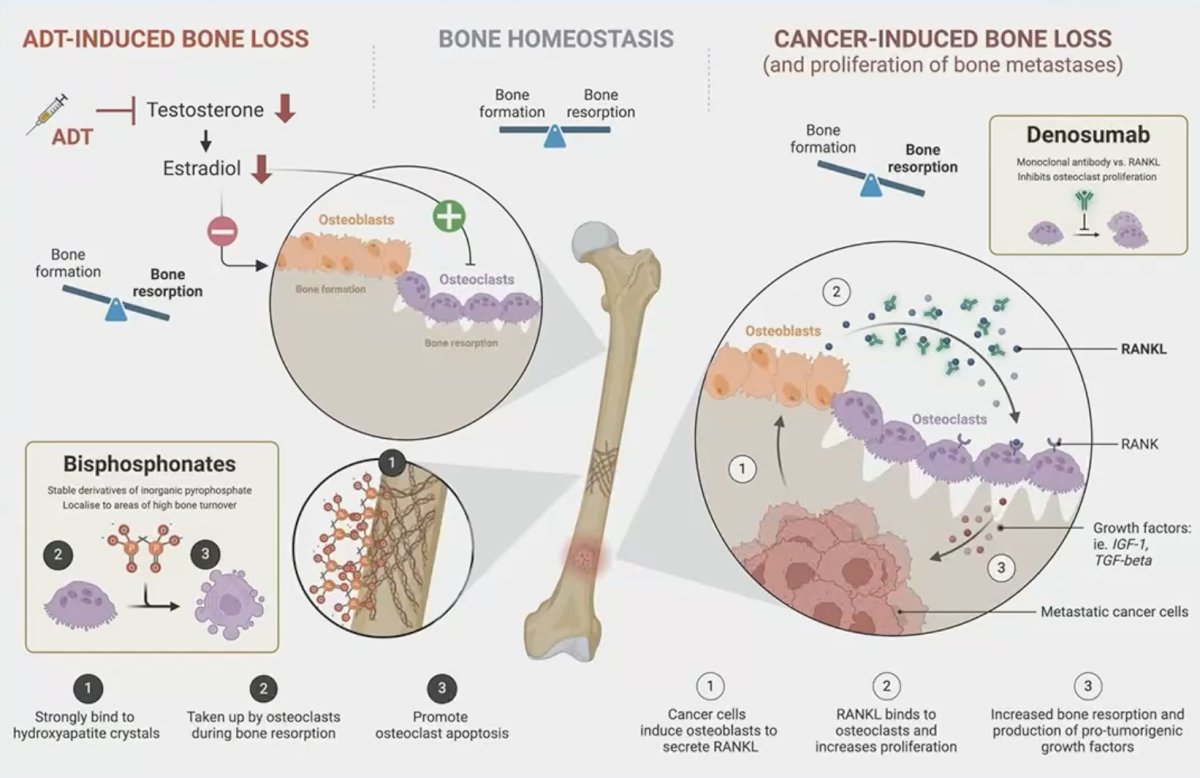
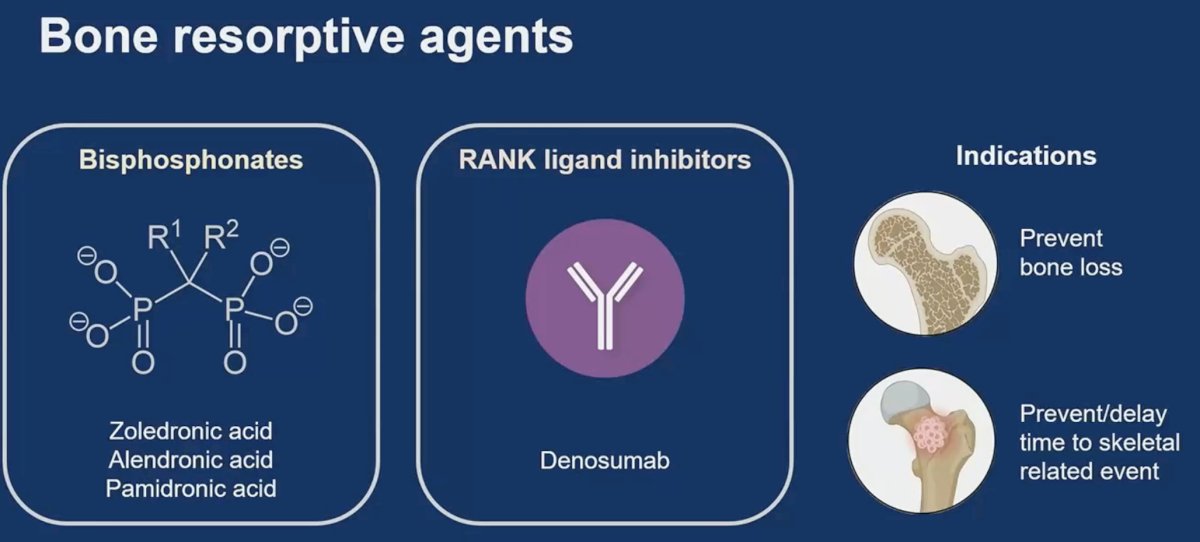
There are numerous mechanisms elucidating bone loss in prostate cancer. Suppression of testosterone via ADT also leads to a decrease in the levels of estrogen, which has an important role in upregulating osteoblast activity and downregulating osteoclast activity. Additionally, cancer cells induce osteoblasts to secrete RANK ligand (RANKL) which binds to osteoclasts and leads to increased bone resorption. Bone protective agents such as denosumab and bisphosphonates target these pathophysiologic/aberrant alterations leading to decreased bone resorption.
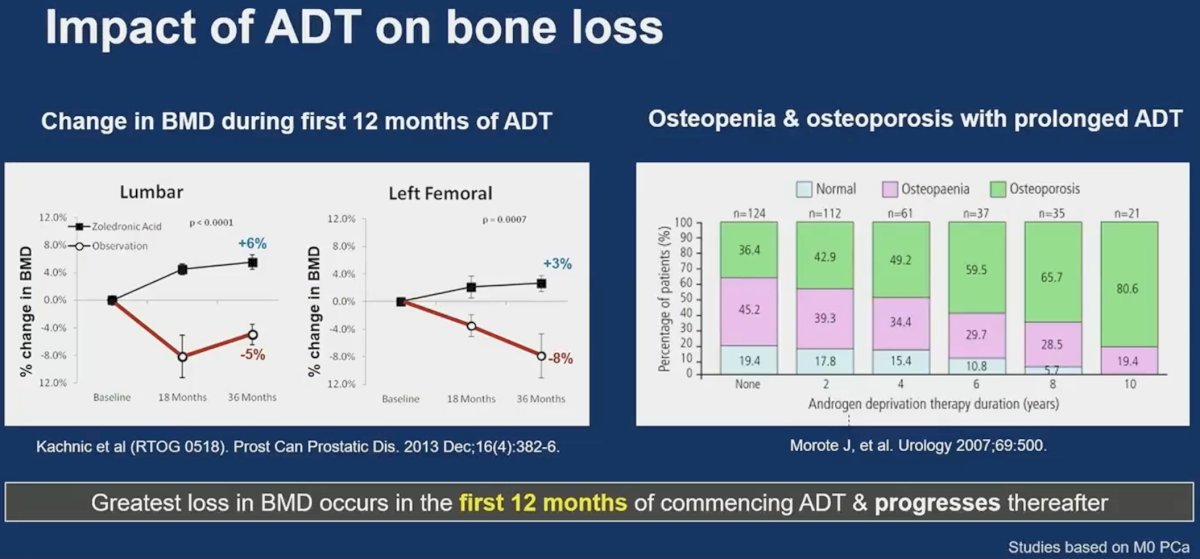
It appears that the greatest losses in bone mineral density occur during the first 12 months of commencing ADT and progresses thereafter.
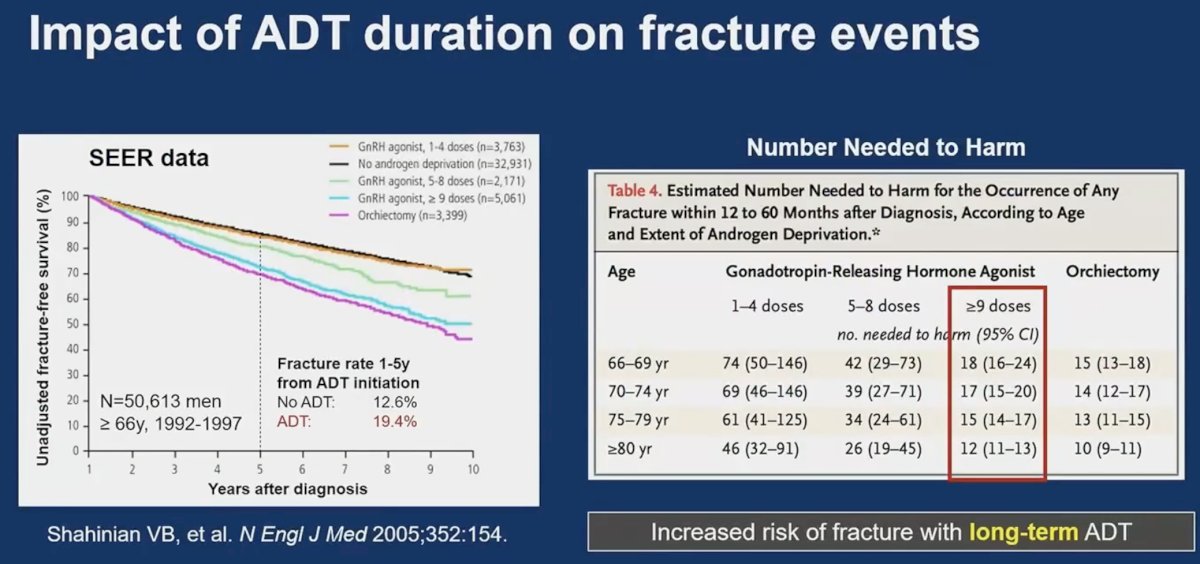
SEER data demonstrate that the fracture risk is higher with longer durations of ADT exposure. It appears that the number needed to harm ranges between 10 and 20, meaning that one in 10–20 patients initiating ADT are likely to suffer a bone fracture from long-term ADT use.
Data from the NPCA-UK suggest that ~40% of advanced prostate cancer patients experience a skeletal-related event by five years.3

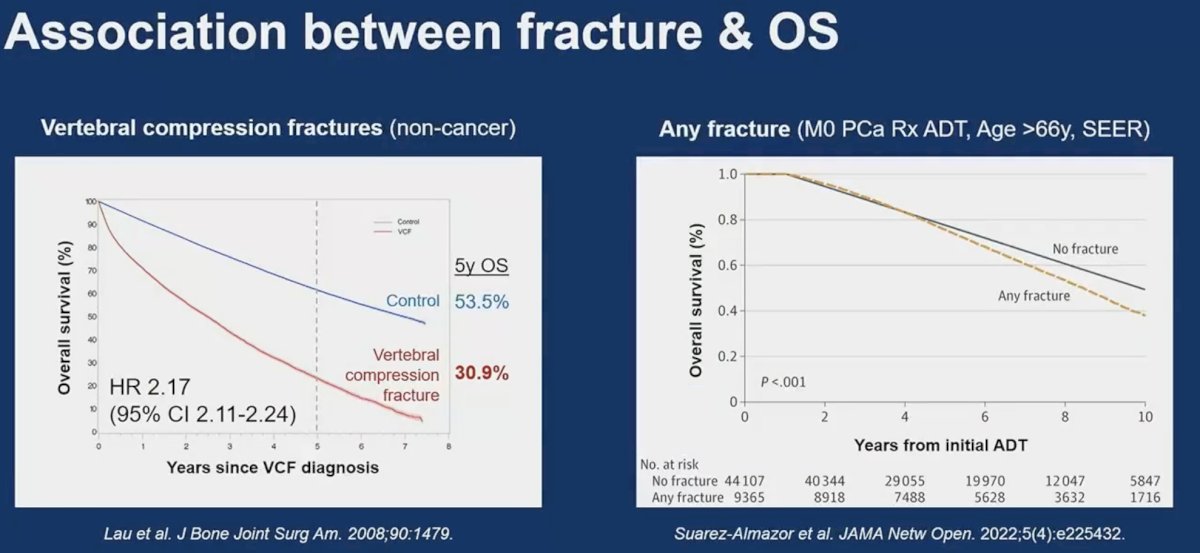
The importance of skeletal fractures is underlined by their strong association with overall survival outcomes. Patients with vertebral compression fractures have a 2.17-fold increased rate of overall mortality.4 Patients with any fracture have an almost 10% lower 10-year overall survival rate.5
Bone resorptive agents have a dual role in preventing bone loss and preventing/delaying time to skeletal-related events.
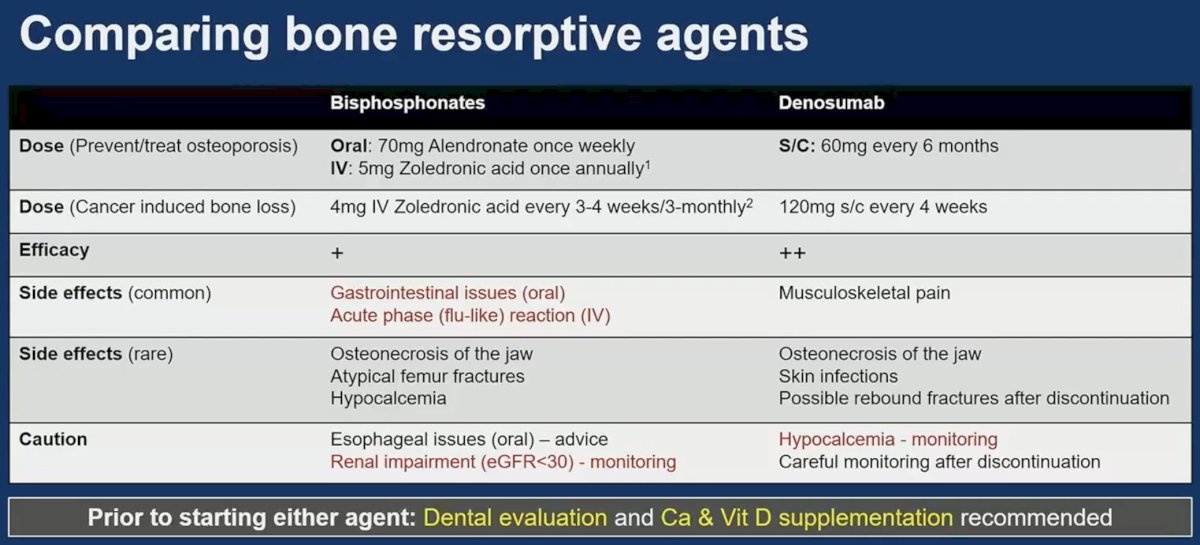
How do these agents (bisphosphonates and denosumab) compare? It appears that denosumab is more efficacious and is associated with a more favorable side effect profile. It is important to note that prior to starting either agent, a dental evaluation and calcium/vitamin D supplementation are recommended, and serial monitoring of renal function/calcium levels should be performed.
Calcium and vitamin D supplementation do not offer sufficient bone protection in ADT-treated prostate cancer patients when used alone. The National Osteoporosis Foundation recommends >1,200 mg of calcium daily and >800 IU of vitamin D daily (total from diet + supplement) for patients receiving bone resorptive agents. Patients should also be encouraged to adopt lifestyle changes, including weight-bearing exercising, smoking cessation, and limiting alcohol consumption.
There are three key trials of zoledronic acid in mHSPC patients: CALGB 90202,6 STAMPEDE ABCE,7 and ZAPCA.8
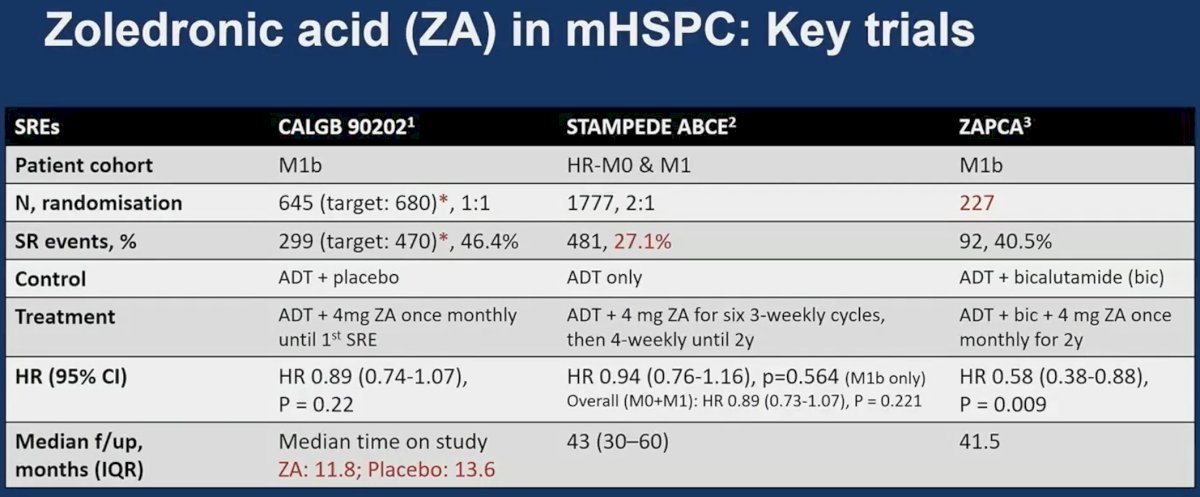
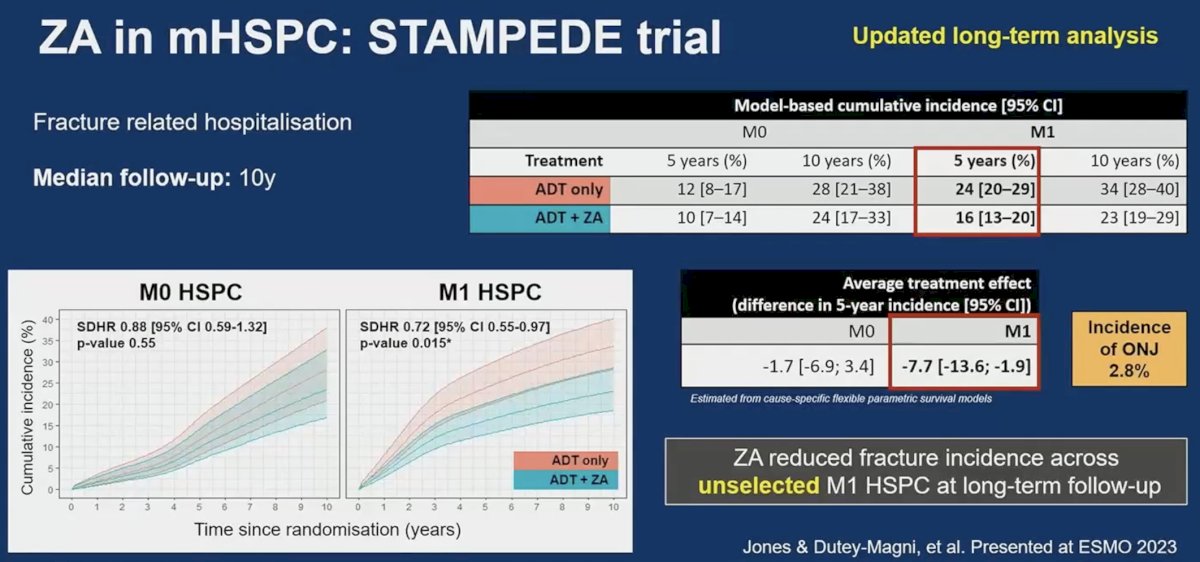
Of these three trials, only ZAPCA demonstrated a significant decrease in the incidence of skeletal-related events. There are however important between-trial differences that may explain the results observed. ZAPCA included only 227 patients compared to 645 and 1,777 in CALGB 90202 and STAMPEDE ABCE, respectively. While CALGB 90202 and ZAPCA only included patients with M1b disease, STAMPEDE ABCE included patients with high-risk, non-metastatic disease. The median follow-up time in CALGB 90202 was only 12 months, which may be limited to evaluate an outcome such as skeletal-related events. In STAMPEDE ABCE, the proportion of patients with skeletal-related events was relatively low (27.1%), which suggests that longer follow-up may have been of value in this trial. Updated analysis of this trial presented at ESMO 2023 suggests that zoledronic acid may actually reduce the incidence of skeletal related events when analysis is limited to patients with M1 disease (7.7 decrease in 5-year fracture incidence).
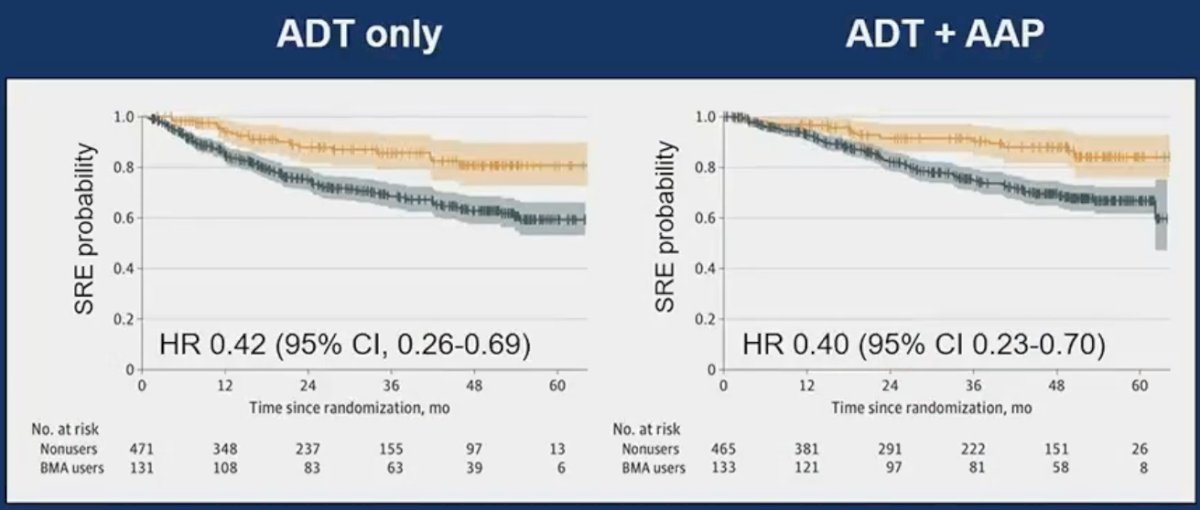
Further evidence to support the use of bone resorptive agents for mHSPC patients is seen from the LATITUDE trial (ADT +/- abiraterone acetate/prednisone), with a post hoc, non-randomized, IPTW-adjusted analysis demonstrating that bone resorptive agents reduced the incidence of skeletal-related events in both ADT-only (HR: 0.42, 95% CI: 0.26–0.69) and ADT + abiraterone-treated patients (HR: 0.40, 95% CI: 0.23–0.70).9
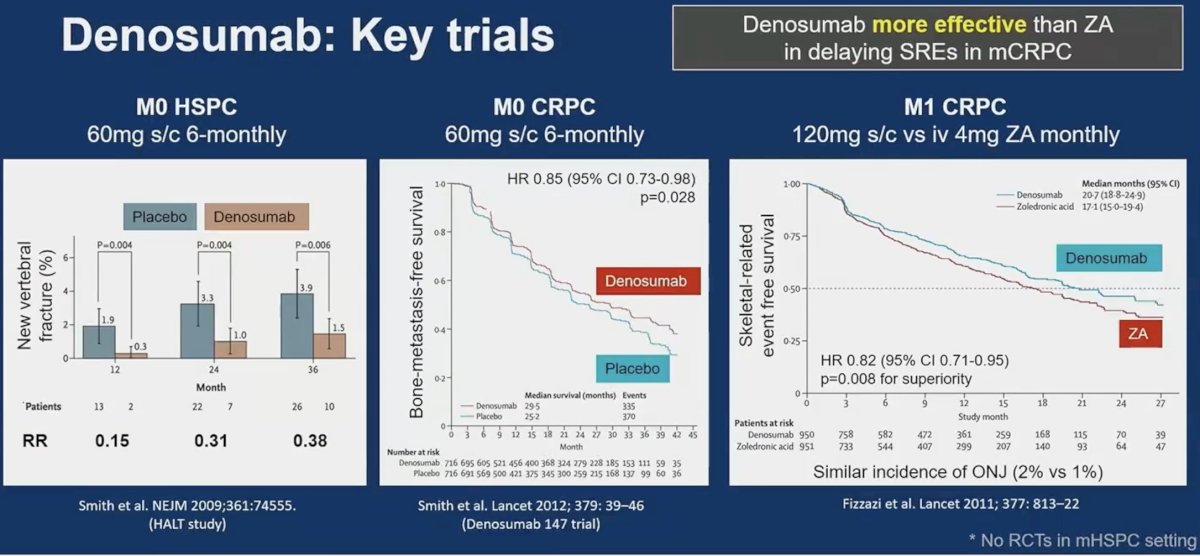
There are three key trials of denosumab in advanced prostate cancer: HALT in M0 hormone-sensitive disease,10 Denosumab 147 trial in M0 CRPC patients,11 and a randomized trial of denosumab versus zoledronic acid in mCRPC patients, which demonstrated that denosumab was superior for delaying skeletal-related events in mCRPC patients (HR: 0.82, 95% CI: 0.71–0.95, p=0.008).12
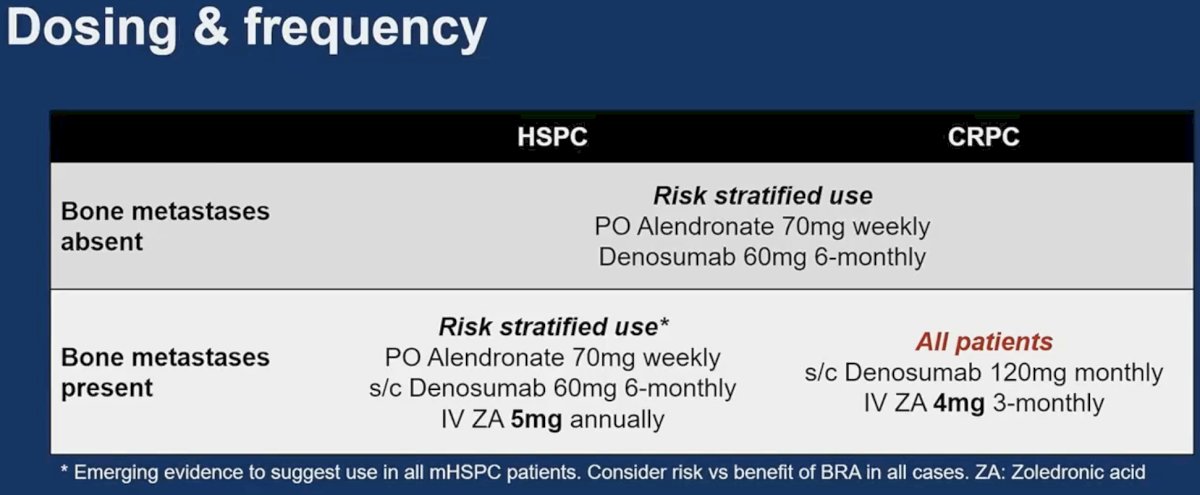
As such, these are the recommendations for the use of bone protective agents based on presence/absence of metastases and presence of castrate sensitive versus resistant disease:
Risk stratification can be performed using:
- Clinical factors, including age, obesity, smoking, alcohol, and steroid use status
- Imaging using serial DEXA scans
- Risk scores, such as FRAX® risk score to predict 10-year fracture risk, which incorporates both clinical and imaging variables

Dr. Sachdeva concluded his presentation with the following take home messages:
- Denosumab and bisphosphonates reduce the risk of fractures and delay skeletal-related events.
- They are appropriate for use in, along with calcium and vitamin D supplementation, in:
- Some high-risk patients with mHSPC
- All mCRPC patients
- Patients should have their serum calcium and renal function monitored. Patients should have regular dental checks given the 1-3% risk of osteonecrosis of the jaw.
- The optimum treatment duration is unknown
- Likely lifelong or when potential risks outweigh benefits
Presented by: Ashwin Sachdeva, MBBS, PhD, FRCS, Division of Cancer Sciences, University of Manchester & Department of Surgery, The Christie Hospital, Manchester, UK.
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024
References:
- Shapiro CL, Van Poznak C, Lacchetti C, et al. Management of Osteoporosis in Survivors of Adult Cancers With Nonmetastatic Disease: ASCO Clinical Practice Guideline. J Clin Oncol. 2019;37(31): 2916-46.
- Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352(2): 154-64.
- Parry MG, Cowling TE, Sujenthiran A, et al. Identifying skeletal-related events for prostate cancer patients in routinely collected hospital data. Cancer Epidemiol. 2019;63:101628.
- Lau E, Ong K, Kurtz S, Schmier J, Edidin A. Mortality following the diagnosis of a vertebral compression fracture in the Medicare population. J Bone Joint Surg Am. 2008;90(7): 1479-86.
- Suarez-Almazor ME, Pundole X, Cabanillas G, et al. Association of Bone Mineral Density Testing With Risk of Major Osteoporotic Fractures Among Older Men Receiving Androgen Deprivation Therapy to Treat Localized or Regional Prostate Cancer. JAMA Netw Open. 2022;5(4): e225432.
- Smith MR, Halabi S, Ryan C, et al. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (alliance). J Clin Oncol. 2014;32(11): 1143-50.
- James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024): 1163-77.
- Kamba T, Kamoto T, Maruo S, et al. A phase III multicenter, randomized, controlled study of combined androgen blockade with versus without zoledronic acid in prostate cancer patients with metastatic bone disease: results of the ZAPCA trial. Int J Clin Oncol. 2017;22(1): 166-73.
- Fukuokaya W, Mori K, Urabe F, et al. Bone-Modifying Agents in Patients With High-Risk Metastatic Castration-Sensitive Prostate Cancer Treated With Abiraterone Acetate. JAMA Netw Open. 2024;7(3): e242467.
- Smith MR, Egerdie B, Toriz NH, et al. Denosumab in Men Receiving Androgen-Deprivation Therapy for Prostate Cancer. N Engl J Med. 2009;361: 745-55.
- Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379(9810): 39-46.
- Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768): 813-22.


