(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured a session on prostate cancer, and a presentation by Dr. Deepak Kilari discussing results of a phase II trial of enzalutamide with 5-alpha reductase inhibitors as an ADT–sparing approach for older men with castration-sensitive prostate cancer (CSPC).
Androgen deprivation is considered the standard of care for men with CSPC. However, with the aging population, the number of older men with prostate cancer who have aging-related conditions are at high risk for adverse events from ADT. 5-alpha reductase inhibitors inhibit the conversion of testosterone to dihydrotestosterone, limiting cell proliferation, and could be synergistic with enzalutamide. In this phase II study, Dr. Kilari and colleagues evaluated enzalutamide and dutasteride or finasteride in lieu of ADT for older men with CSPC who are at risk of adverse events from ADT. Eligible patients were as follows:
- ≥65 years
- Deemed “not fit” by geriatric assessment or at high risk for side effects from ADT as determined by the treating physician
- Had metastatic (M1) or non-metastatic (M0) CSPC with a PSA doubling time ≤9 months
- Testosterone > 50ng/dl
Enzalutamide 160 mg daily and dutasteride 0.5 mg daily or finasteride 5 mg daily were administered until disease progression per Prostate Cancer Clinical Trials Working Group 2 guidelines. The primary study endpoint was PSA progression-free survival. Key secondary endpoints included time to PSA nadir, absolute PSA nadir, and evaluation of safety, and toxicity of study treatments per CTCAE V4.0. From a statistical standpoint, The corresponding 1-year PSA progression-free survival times under null and alternative hypotheses are assumed to be 1.44 and 3.04 years, and the corresponding 1-year PSA progression-free survival rates are 50% and 72%, respectively. A sample size of 40 patients has 82% power, accounting for a 5% dropout.
Overall, there were 43 men enrolled in the study. At study entry, subjects had the following baseline characteristics:
- Median age was 78 years (range: 66-94)
- 93% had ECOG 0-1
- 63% had M1 CSPC with 30% of them having high volume disease per CHAARTED criteria
- 23% had Gleason ≥ 8 CSPC
- Median PSA was 11.4 ng/ml (range: 2-145)
- Median testosterone level was 342 ng/dl (range: 56-639)
- 56% of men had osteopenia or osteoporosis
Baseline geriatric assessment at study entry showed that (i) 18.6% had Instrumental Activity of Daily Living impairment, (ii) 9.8% had recent falls, (iii) 52.4% had Short Physical Performance Battery impairment, (iv) 40.5% had Older American Resources and Services (OARS) physical health and 31% had OARS medical social support impairments, (v) 11.6% had Geriatric Depression Scale impairments, (vi) 65.9% had either Blessed Orientation-Memory-Concentration or Montreal Cognitive Assessment impairments, and (vii) 34.9% had Vulnerable Elders Survey-13 impairments.
At a median follow-up of 5.96 years (range: 0.12 to 7.56 years), subjects were censored at treatment end date or December 1, 2022. Among the 43 enrolled participants, nine had events at the time of censoring, four had disease progression, and five had died. A total of 34 men (79.1%) were alive at the time of censoring. There were 23 patients (53%) that remained on study treatment and the median PSA progression-free survival has not been reached for the entire group (stratified metastatic versus no metastatic disease):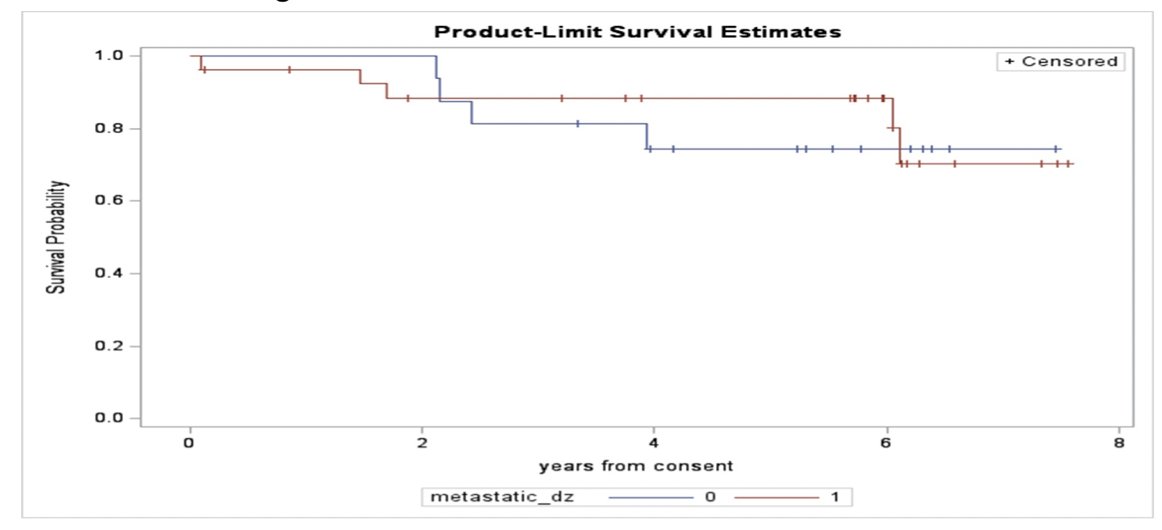
The median time to PSA nadir was 8.1 months (range: .4-78.3), with median PSA nadir at 0.02 ng/ml (range 0-2.75), and 98% having ≥90% PSA decline: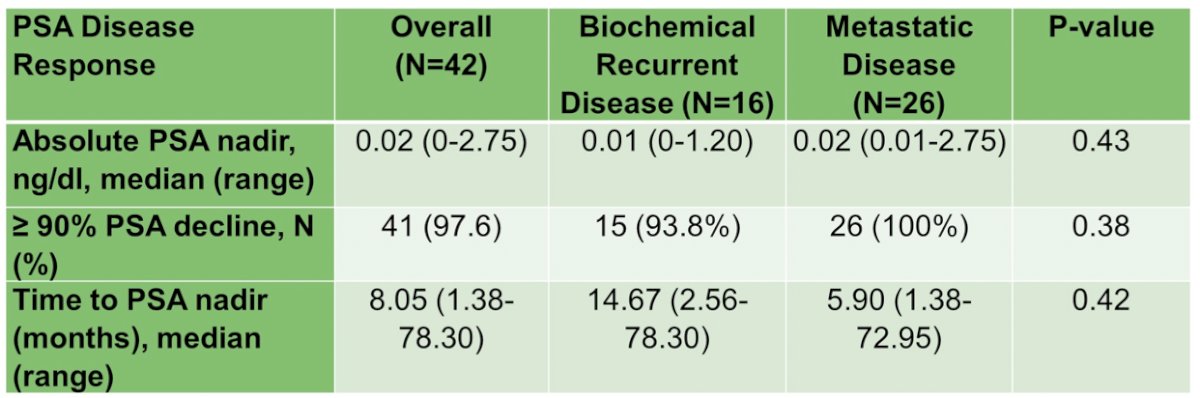
The five most common any-grade adverse events were fatigue (88%), gynecomastia (72%), hot flashes (42%), hypertension (40%), and dyspnea (40%). No subject had treatment-related Grade 4 or 5 adverse events. One patient had grade 3 myocardial infarction possibly attributed to study drugs. There were four (9.3%) patients who withdrew treatment, 21% who held treatments, and 30.2% who reduced dose of treatment due to adverse events.
Specific to the comprehensive geriatric assessment data, at baseline, 31 (72%) of patients had impairments in >= 2 CGA domains, and 19% reported impairments in their ability to perform instrumental activities of daily living independently. Overall, 10% of patients reported recent falls, 53% had impairments in physical function as measured by the short physical performance battery test, 64% had cognitive impairment detected by the Montreal Cognitive Assessment, and 98% of patients reported polypharmacy (> 5 medications). With regards to changes in CGA domains with treatment, only the prevalence of impairment in instrumental Activities of Daily Living and OARS physical health showed a statistically significant worsening from baseline to week 61.
Dr. Kilari concluded his presentation discussing results of a phase II trial of enzalutamide with 5-alpha reductase inhibitors as an ADT–sparing approach for older men with CSPC by noting that the combination treatment with enzalutamide and dutasteride/finasteride appears to have clinical efficacy for older patients with CSPC who are at high risk for adverse events from ADT
Presented by: Deepak Kilari, MD, Oncologist, The Medical College of Wisconsin, Milwaukee, WI
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.


