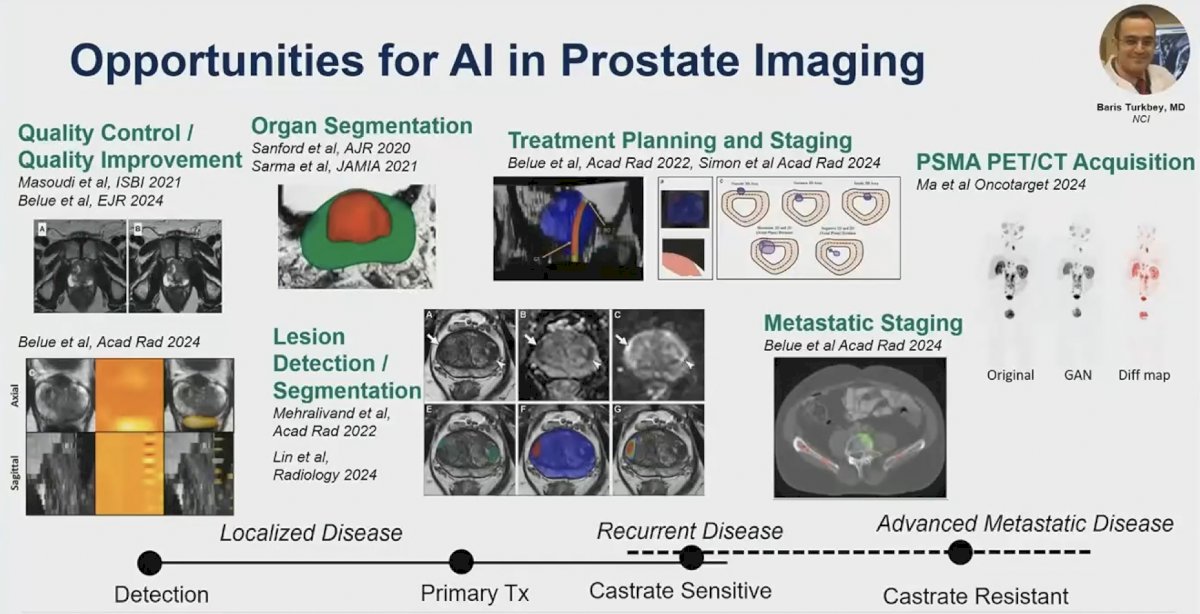
(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured a session on applications of artificial intelligence in prostate cancer care, and a presentation by Dr. Stephanie Harmon discussing innovation and potential for diagnostic imaging in prostate cancer. There are many opportunities for artificial intelligence in prostate imaging, including:
- Quality control/quality improvement
- Organ segmentation
- Lesions detection/segmentation
- Treatment planning and staging
- Metastatic staging
- PSMA PET/CT acquisition
With regards to multiparametric MRI and artificial intelligence, mpMRI is an essential step in the prostate cancer diagnostic pathway, providing assistance with biopsy guidance, active surveillance, and treatment planning. The Prostate Imaging Reporting and Data System (PI-RADS) was developed to standardize image acquisition techniques and interpretation of prostate mpMRI. However, site-to-site quality variability has reduced the effectiveness of prostate mpMRI specifically for:
- Image quality: failure to comply with PI-RADS technical requirements, but compliance alone does not ensure higher-quality image
- Interpretation quality: inter- and intra-reader variability, especially in non-academic centers
The following figure highlights the variability in site-specific PPV for PI-RADS:1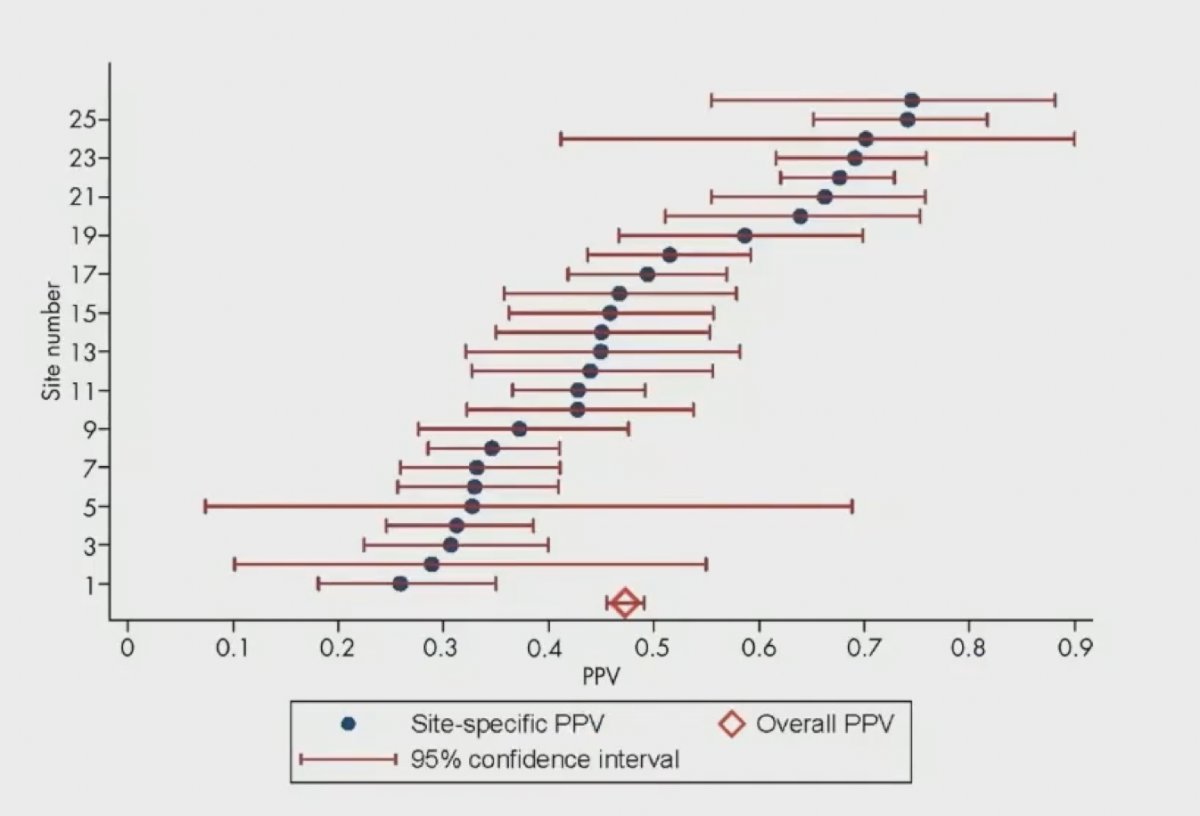
Given these issues, public artificial intelligence challenges and datasets have accelerated academic research, and clinical adoption of mpMRI has further accelerated translation. How do we translate the diagnostic workflow into clinical practice?
- Development of a pipeline that matches the clinical workflow:
- Was the population for training pre-selected?
- Has validation been completed for end-to-end processing pipelines?
- Are the images appropriate for artificial intelligence use?
- Technical translation needs:
- Needs to be online and fast (ie. in PACS)
- It needs to be secure
- It needs to be easy to interpret
- Needs to support downstream clinical workflow
In 2022, Mehralivand and colleagues developed a bi-parametric mpMRI (T2W, ADC, high b-value) segmentation and classification algorithm from 1,390 patients with the objective of detecting intra-prostatic lesions of the prostate according to PI-RADS guidelines.2 Performance was then compared to the literature, with ~60% sensitivity compared to the expert, a mean Dice similarity coefficient of 0.359, and a low false positive rate (median <1 per patient). As follows is a sample of a visual result for algorithm prediction and visualization: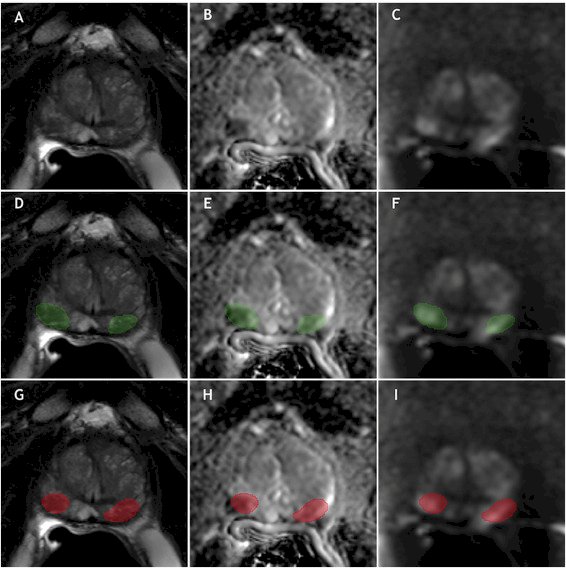
This model was recently validated in 658 patients, maintaining performance compared to an expert radiologist.3 Deployment in the clinical workflow may look as follows: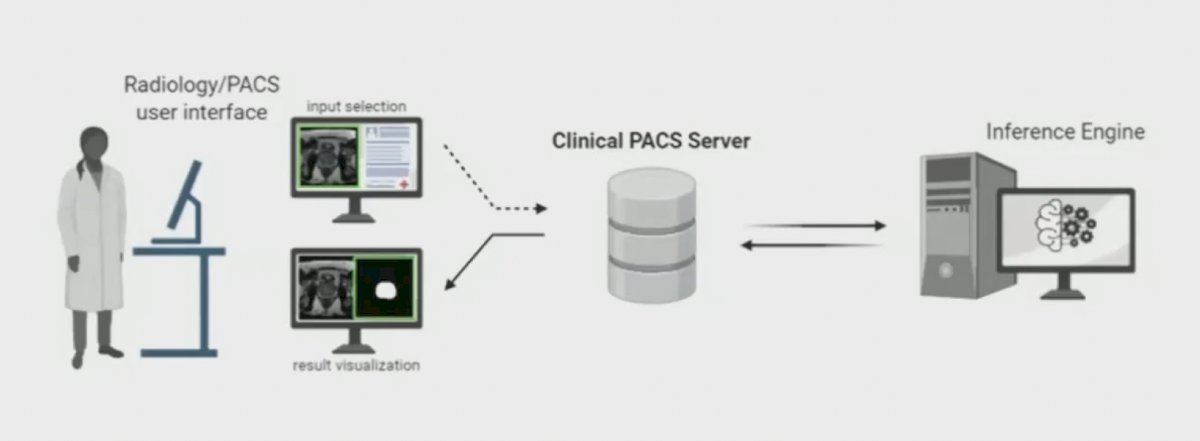
First, is a data induction stage, which is followed by automated processing, configuration, and visualization: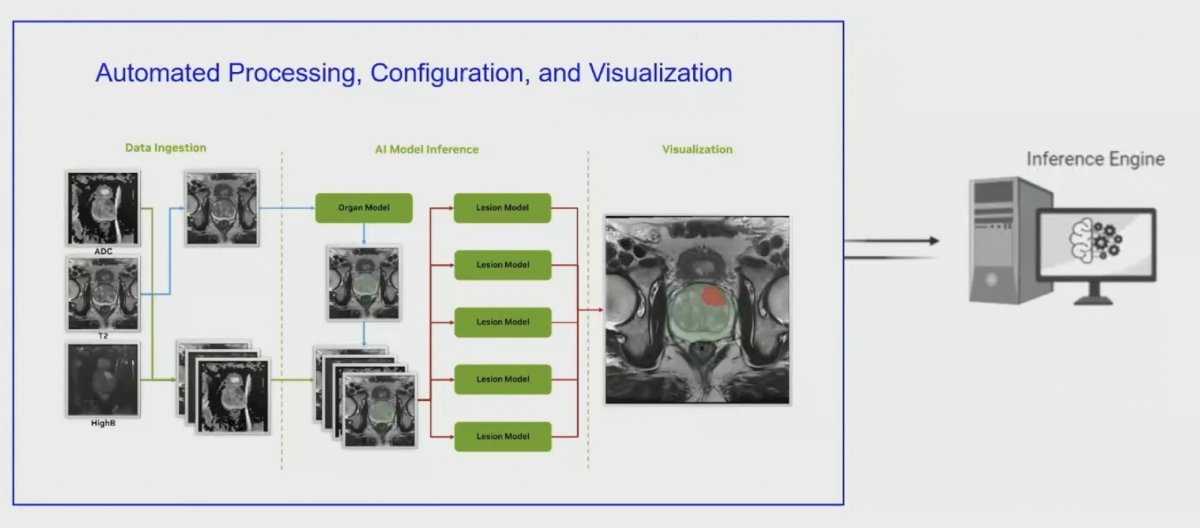
Multi-reader studies evaluating the impact of artificial intelligence indicate that radiologist interaction can bias efficacy. For example, the attention method improves sensitivity of uncommon/”invisible” cancer lesions, but only in experienced readers.
Artificial intelligence-based methods for prostate MRI are typically trained and tested using data with ideal scanning and anatomical conditions, (i) which may lead to model overfitting, (ii) could result in poor generalizability, and (ii) necessitates stress-testing models in real-world cases and data from external centers. Prostate organ segmentation by three independent artificial intelligence models in 684 challenging scans showed that the performance of all models was negatively impacted by prostate volume and poor signal quality (p < 0.01).4 Shape-based factors influenced DL models (p < 0.001), while signal factors influenced all (p < 0.001):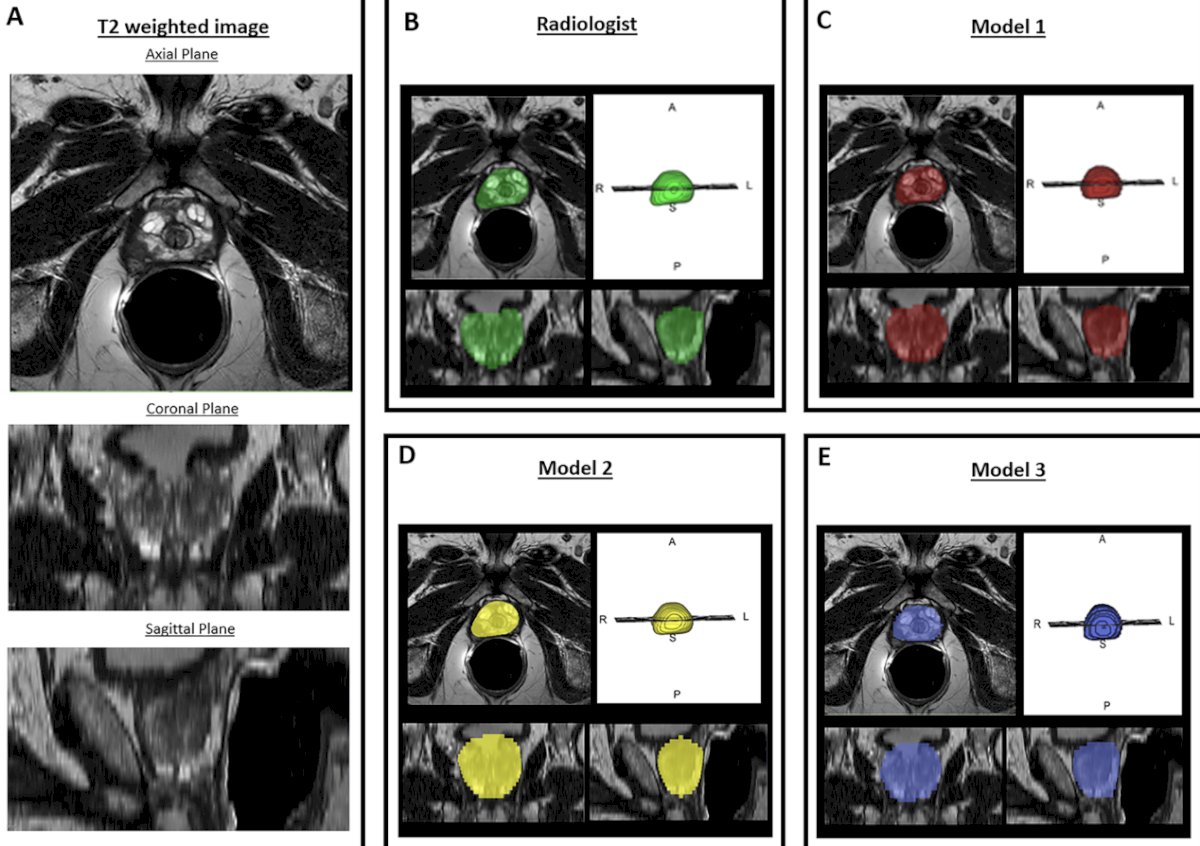
What happens after prior treatment? Among 62 patients having prostate lesions assessed after prostate radiotherapy, the artificial intelligence model performance was lower than the prospective radiology interpretation (artificial intelligence: 76.1% vs radiologist: 91.3%, p = 0.02) and lesion level (artificial intelligence: 71.4% vs radiologist: 87.5%, p = 0.01):5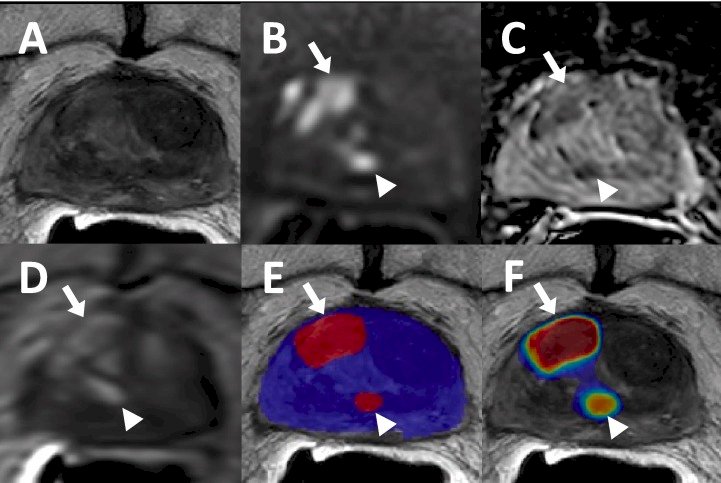
Opportunities for future improvements include federated learning and artificial intelligence for image quality. In a study by Sarma et al.6 in 2021, a federated learning model exhibited superior performance and generalizability to the models trained at single institutions when evaluated on an outside challenge dataset: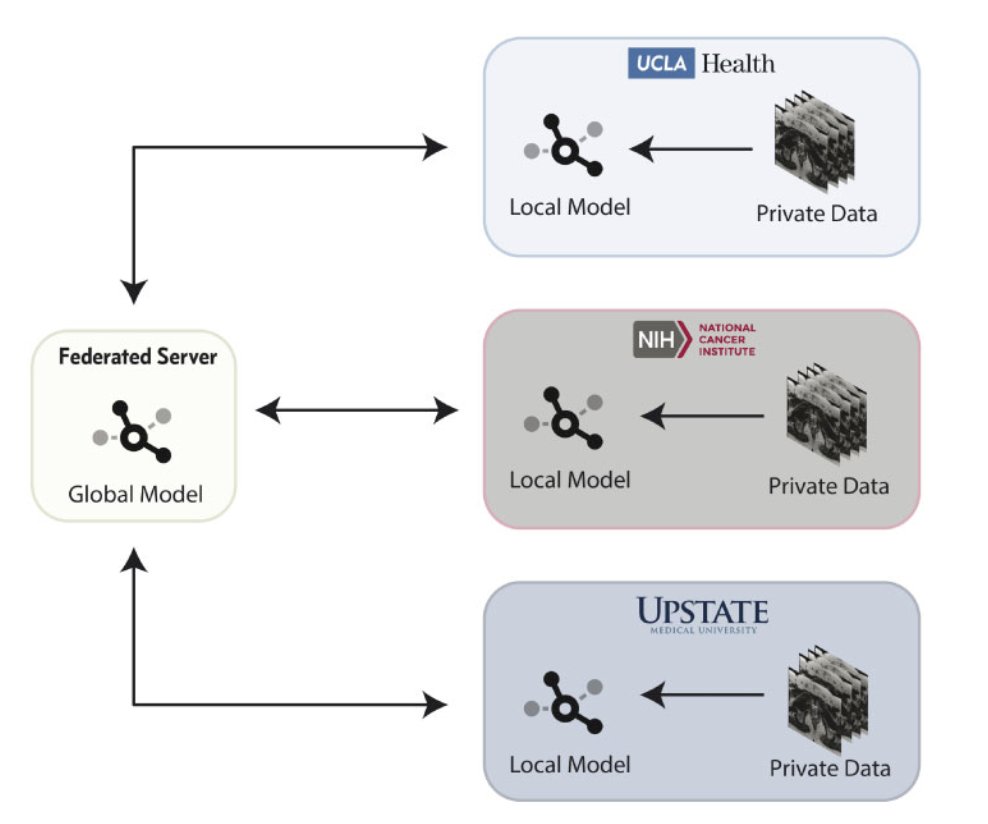
Dr. Harmon then moved to discussing advanced disease imaging. Diagnostic imaging (CT, MRI, bone scan) is critical for the detection and longitudinal monitoring of advanced disease and systemic therapies. Recently, PSMA PET/CT demonstrates high diagnostic accuracy compared to conventional staging in recurrent and castrate-sensitive settings. Despite its high diagnostic accuracy compared to conventional imaging, PSMA PET/CT still suffers from inter-reader variability, labor-intensive reads, and multiple isotopes/radiotracers. Because of this, there are FDA-cleared commercial algorithms available. Challenges for artificial intelligence include training populations (recurrence, castrate sensitive, castrate-resistant), as well as having multiple isotopes available.
Treatment monitoring comprises a large body of images obtained in the advanced prostate cancer setting, given that there are numerous systemic therapies available through the course of advanced to late-stage prostate cancer. The role for diagnostic imaging may be treatment-dependent:
- Traditional RECIST was developed on conventional imaging
- Artificial intelligence may be used for longitudinal disease quantification
- Targeted-agent therapeutics
Dr. Harmon concluded her presentation discussing innovation and potential for diagnostic imaging in prostate cancer with the following take-home messages:
- There are many clinical opportunities for artificial intelligence in prostate cancer imaging
- Artificial intelligence needs may vary by modality and disease stage
- Validation is key
- There are technical needs for translation
- Needs to be secure and connected to the clinical workflow
- Needs to be easy to interpret and user-friendly
- More investigation is needed in implementation science
Presented by: Stephanie A. Harmon, PhD, Staff Scientist, Molecular Imaging Program, National Cancer Institute, Bethesda, MD
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
References:
- Westphalen AC, McCulloch CE, Anaokar JM, et al. Variability of the Positive Predictive Value of PI-RADS for Prostate MRI across 26 Centers: Experience of the Society of Abdominal Radiology Prostate Cancer Disease-focused Panel. Radiology. 2020 Jul;296(1):76-84.
- Mehralivand S, Yang D, Harmon SA, et la. A cascaded deep learning-based artificial intelligence algorithm for automated lesion detection and classification on biparametric prostate magnetic resonance imaging. Acad Radiol. 2022 Aug;29(8):1159-1168.
- Lin Y, Yilmaz EC, Belue MJ, et al. Evaluation of a cascaded deep learning-based algorithm for prostate lesion detection at biparametric MRI. Radiology. 2024 May;311(2):e230750
- Johnson LA, Harmon SA, Yilmaz EC, et al. Automated prostate gland segmentation in challenging clinical cases: Comparison of three artificial intelligence methods. Abdom Radiol. 2024 May;49(5):1545-1556.
- Yilmaz EC, Harmon SA, Belue MJ, et al. Evaluation of a deep learning-based algorithm for post-radiotherapy prostate cancer local recurrence detection using biparametric MRI. Eur J Radiol. 2023 Nov;168:111095.
- Sarma KV, Harmon S, Sanford T, et al. Federated learning improves site performance in multicenter deep learning without data sharing. J Am Med Inform Assoc. 2021 Jun 12;28(6):1259-1264.


