(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting featured a session on penile cancer, and a presentation by Dr. Fernando C. Maluf discussing results of HERCULES, a phase 2 trial of pembrolizumab + platinum-based chemotherapy as first-line systemic therapy in advanced penile cancer. Penile squamous cell carcinoma is a rare disease with an incidence of 38,000 new cases per year globally, but with up to 10x higher incidence in low-income countries (ie. Africa, Asia, Latin America). There have been no improvements over the last six decades, with platinum-based chemotherapy being the standard of care first line treatment for advanced penile squamous cell carcinoma. Still, advanced penile squamous cell carcinoma has a poor prognosis (overall survival 6-7 months) with limited treatment options. Previously, doublet platinum based chemotherapy has been assessed, but with limited efficacy (objective response rate 20-30%, progression free survival of 3 months). Triplet platinum based chemotherapy resulted in objective responses of 38%-45%, but at the cost of high toxicity (33%-46% neutropenia Grade 3-4, 25% sepsis rate), and thus high rates of treatment discontinuation. Immune checkpoint inhibitors have been associated with improved efficacy in different types of malignancies, however the benefit in penile squamous cell carcinoma is uncertain.
HERCULES (LACOG 0218) is a phase II, single arm trial evaluating pembrolizumab + platinum-based chemotherapy as first line treatment in advanced penile squamous cell carcinoma. Patients with metastatic or locally advanced disease (recurrent or TanyN3M0 or T4NanyM0) not amenable to curative-intent therapy received 5-FU 1000 mg/m²/day IV D1-D4 + cisplatin 70mg/m² (or carboplatin AUC 5) IV D1 + pembrolizumab 200mg IV D1 every 3 weeks for 6 cycles, followed by pembrolizumab 200mg IV every 3 weeks up to 34 cycles. The trial design is as follows:
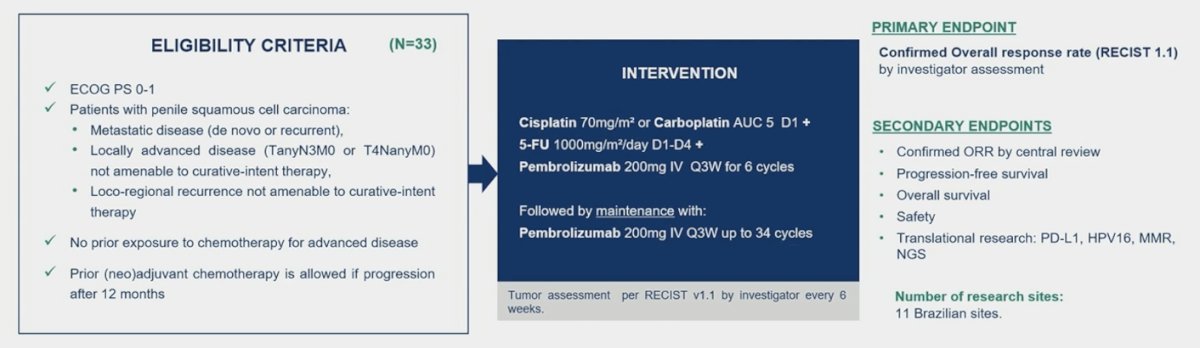
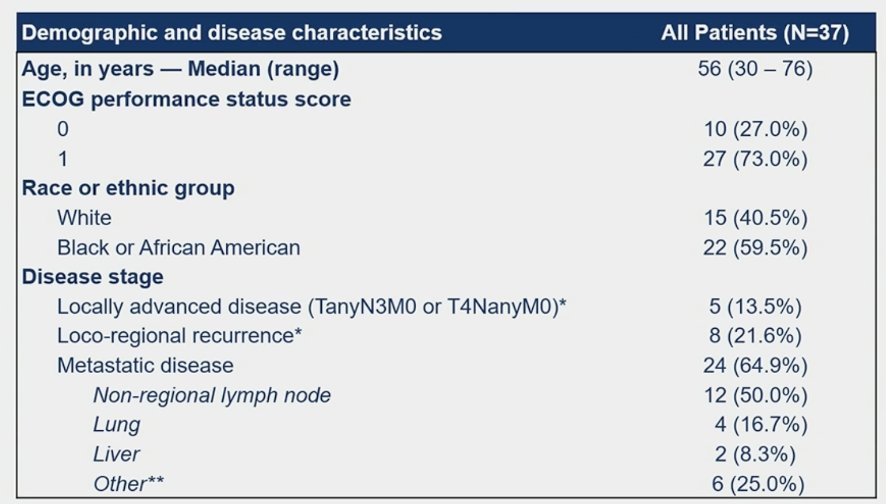
The primary endpoint is confirmed overall response rate assessed by investigator according to RECIST 1.1. Considering a drop-out rate of 10%, 33 patients were required to reject the null hypothesis that objective response rate is 20% or less, if the true objective response rate is 40% (two-sided alpha level of 0.10, power 78.5%). From August 2020 to December 2022, 37 patients were enrolled in 11 Brazilian centers and 33 patients were eligible for efficacy analysis. The median age was 56 years (range: 30-76), 64.9% of patients had metastatic disease, 21.6% had recurrent disease, and 13.5% had locally advanced disease:
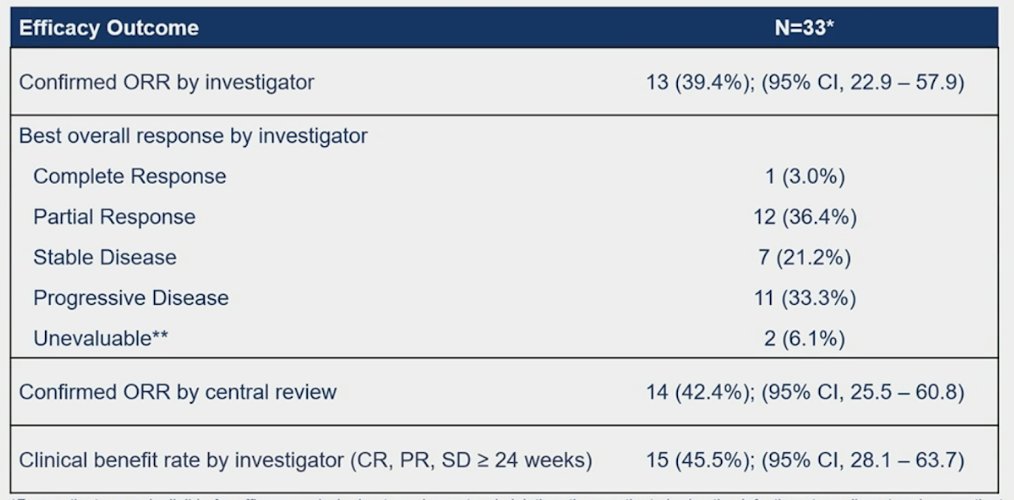
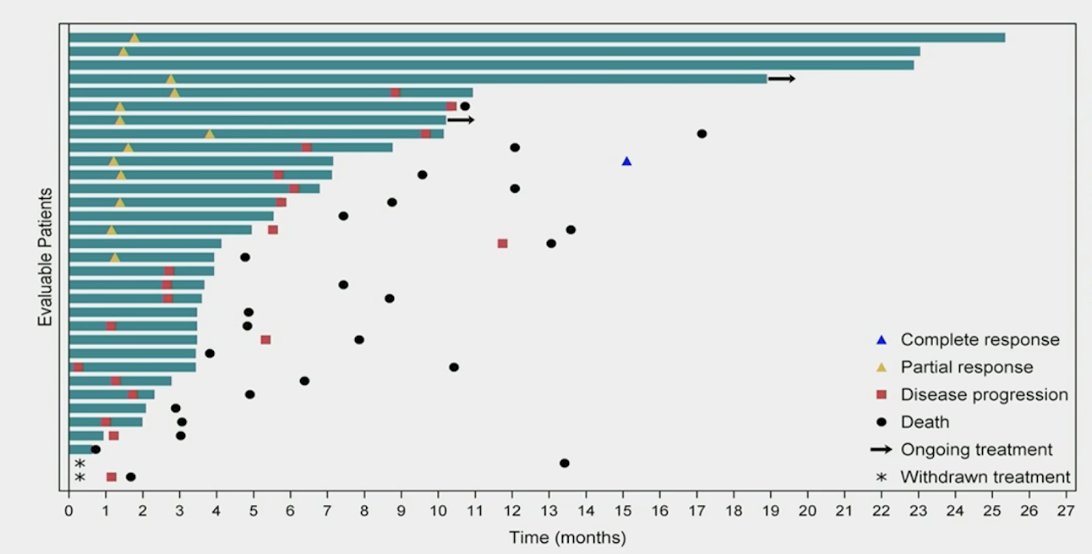
The confirmed overall response rate by investigator was 39.4% (95% CI 22.9-57.9%), with 1 complete response and 12 partial responses:
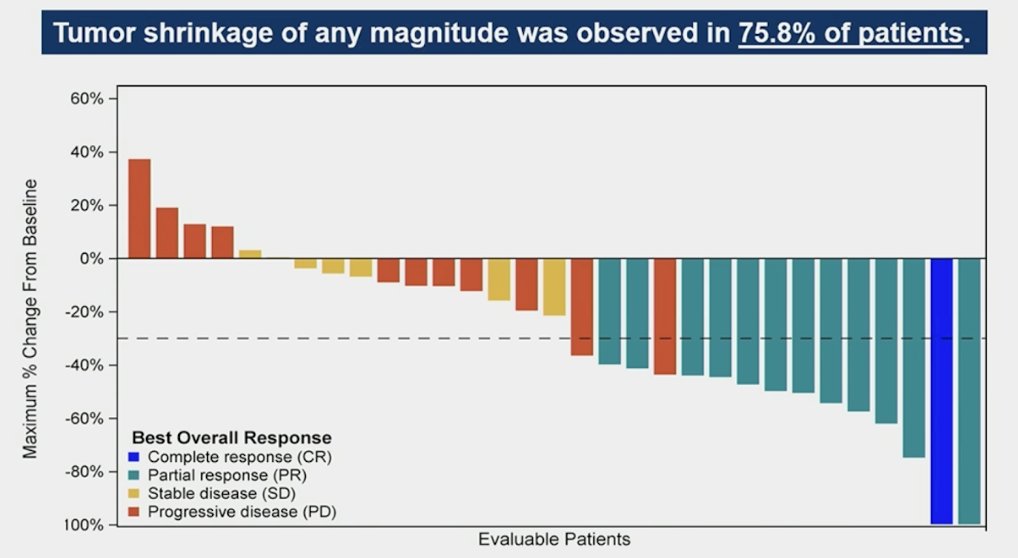
Tumor shrinkage of any magnitude was observed in 75.8% of patients:
The median duration of response was 5.9 months (95% CI 4.4-9.0), the median time to response was 1.4 months (95% CI 1.3-1.8), and the median follow-up was 24.0 months (95% CI 13.5-26.4):
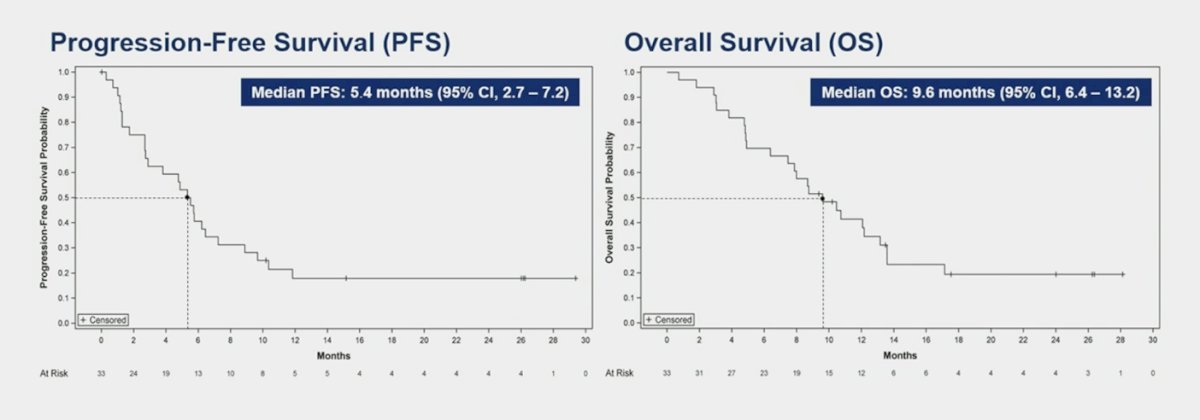
The median progression free survival was 5.4 months (95% CI 2.7-7.2) and the median overall survival was 9.6 months (95% CI 6.4-13.2):
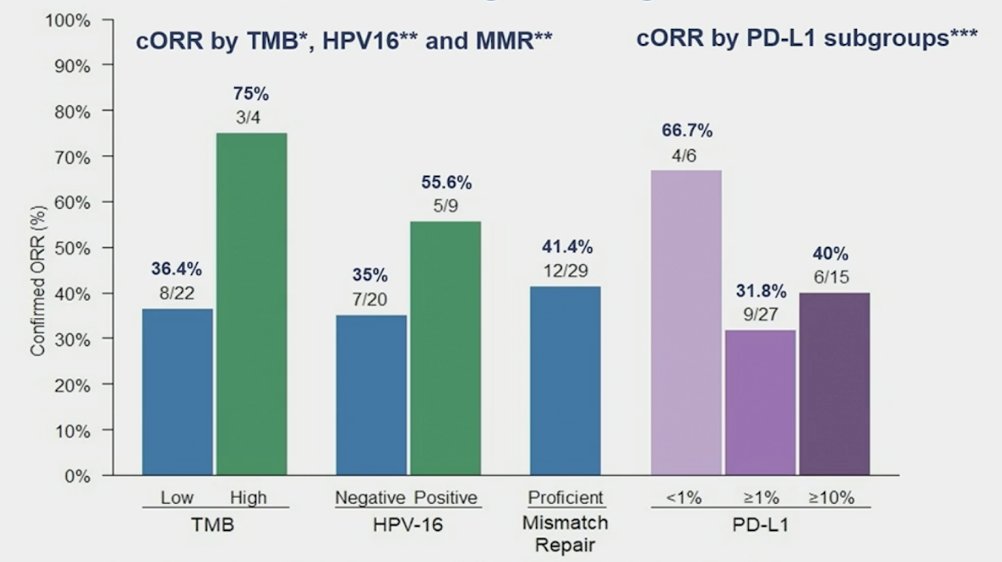
In an exploratory biomarker analysis, confirmed overall response rate investigator according to PD-L1 status was 66.7% in CPS 0% versus 31.8% in CPS ≥ 1%. Confirmed overall response rate investigator according to TMB status was 75% in high versus 36.4% in low. Finally, confirmed overall response rate investigator according to HPV16 status positive was 55.6% versus negative 35.0%:
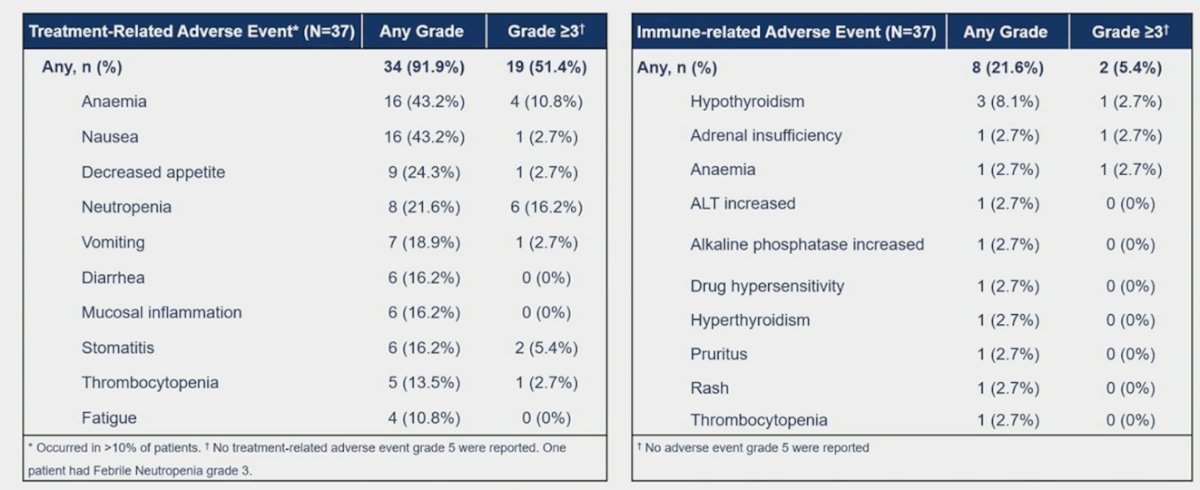
The most frequent genomic alterations detected by NGS were: TP53 (57.1%), CDKN2A (51.4%), and TERT (31.4%). Treatment-related adverse event rate of any grade was 91.9% and grade 3-4 was 51.4%. Ten patients experienced grade 5 adverse events, none of them related to study treatment. Immune-related adverse events of any grade were 21.6% and grade 3-4 was 5.4%. Overall, 10.8% of patients discontinued treatment due to adverse events:
Dr. Maluf concluded his presentation discussing results of HERCULES with the following take home messages:
- HERCULES met its primary endpoint of confirmed objective response rate of 39.4% by investigator assessment, which increased to 42.4% by central review
- HPV16 positive patients had a confirmed objective response rate of 55.6% and TMB-high 75%, which are potential biomarkers for efficacy with chemotherapy + immune checkpoint inhibitors in advanced penile squamous cell carcinoma
- This is the first trial to demonstrate the efficacy of immune checkpoint inhibitors in advanced penile squamous cell carcinoma with a manageable safety profile
- Pembrolizumab + platinum based chemotherapy is a new treatment option for advanced penile squamous cell carcinoma
Presented by: Fernando C. Maluf, Hospital Beneficência Portuguesa de São Paulo and Hospital Israelita Albert Einstein, Sao Paulo, Brazil
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.


