The definition of BCG-unresponsive high-risk NMIBC is as follows:
- BCG refractory
- Stage progression at 3 months after adequate BCG induction (high-grade T1 at 3 months after initial CIS or high-grade Ta)
- Persistent high-risk NMIBC at 6 months despite adequate BCG
- BCG relapsing – recurrence of high-risk NMIBC after the patient achieves a disease-free state within 12 months after adequate BCG therapy
BCC unresponsive CIS will persist if not treated, and because of the high risk of disease progression, radical cystectomy is a recommended standard option for patients with BCG-unresponsive NMIBC. However, many patients are ineligible or decline cystectomy, thus there is an urgent need for novel therapies to reduce the risk of recurrence and preserve the bladder.
Previously it has been shown that the PD-1 inhibitor pembrolizumab has durable antitumor activity in patients with metastatic urothelial carcinoma, both in the first1 and second line2. Based on studies showing an upregulation of the PD-1 pathway among patients with BCG-resistant NMIBC, there are signals to suggest that anti-PD-1 inhibitors, such as pembrolizumab, may be efficacious in these patients. Presented at last year’s ESMO 2018 Congress, the first results of KEYNOTE-057 were reported, suggesting that patients with BCG-unresponsive NMIBC derive a 38.8% complete response rate at 12.4 weeks of follow-up.
During the Challenges and Advances in Perioperative and Local Therapy for Urothelial Carcinoma session at GU ASCO 2019, Dr. Arjun Balar presented updated/interim results of KEYNOTE-057, NCT02625961 for patients with carcinoma in situ (CIS) with or without papillary tumors (cohort A).
KEYNOTE-057 is a single-arm phase 2 study for patients with BCG-unresponsive NMIBC who were unwilling or unfit to undergo radical cystectomy. Specific to this analysis were patients with histologically confirmed high-risk, BCG-unresponsive CIS with or without papillary disease, who received adequate BCG therapy and received pembrolizumab 200 mg Q3W for 24 months or until recurrence, progression, or unacceptable toxicity. Patients with high-risk NMIBC or progressive disease during treatment were required to discontinue pembrolizumab. The primary endpoint for this study was complete response rate and key secondary endpoint for the study were the duration of response and safety. The study design is as follows:
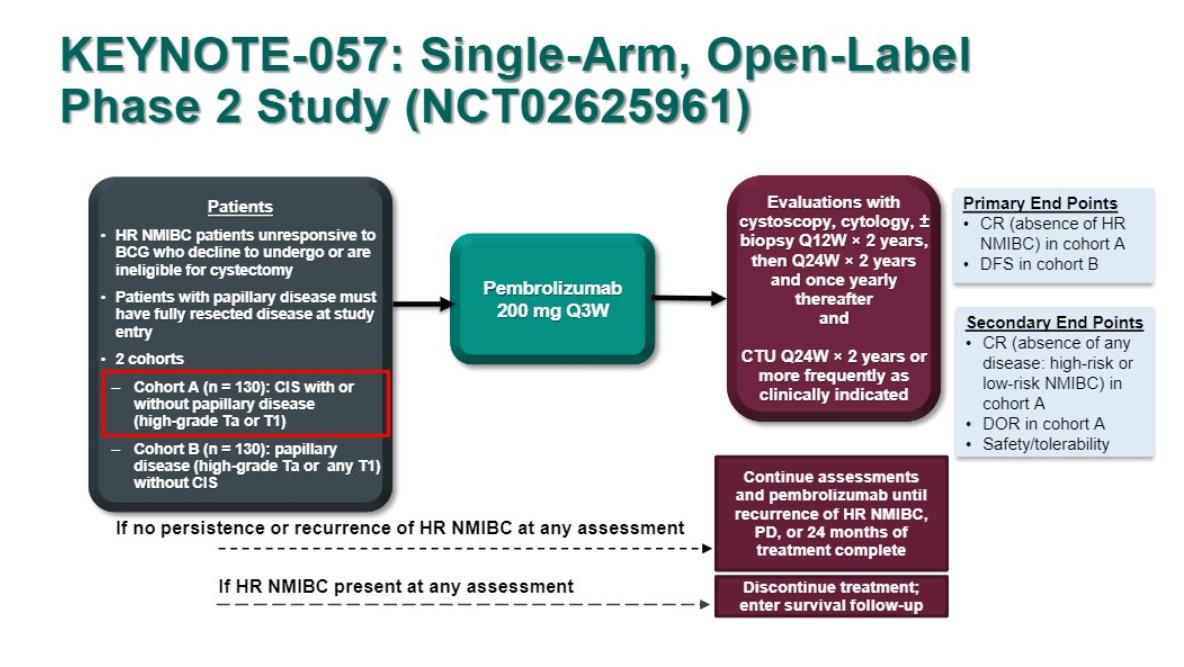
For this analysis, there were 102 patients who met inclusion criteria. The median age was 73 years, 63.7% of patients had CIS alone, and the median number of prior BCG instillations was 12 (range 6.0-45.0). The 3-month complete response rate was 40.2% (95%CI 30.6%-50.4%) by central assessment. Furthermore, among 41 patients who achieved a complete response at 3 months, 58.5% maintained a complete response at last follow-up, which was a median 16.7 months (range 5.9-28.2 months). Impressively, 75% of patients had a complete response duration of ≥6 months and 53% of ≥9 months. Among the 41 patients with complete response at 3 months, 15 patients (36.6%) experienced recurrent NMIBC after their complete response, with none of these patients progressing to muscle-invasive or metastatic disease. Treatment-related adverse events occurred in 66 (64.7%) patients, which was most frequently pruritus (10.7%), fatigue (9.7%), diarrhea (8.7%), hypothyroidism (5.8%), and maculopapular rash (5.8%). Only 13 patients (12.7%) suffered Grade 3/4 treatment-related adverse events, and one death was considered treatment-related (colitis in a patient inadequately treated with steroids). The authors reported that the immune-mediated adverse event rate was 18.4%.
The updated results of KEYNOTE-057 among patients with CIS suggest that pembrolizumab has encouraging activity in patients with high-risk, BCG-unresponsive CIS with or without papillary tumors. Furthermore, the safety profile is consistent with that of previous experience using pembrolizumab for NMIBC. A phase 3 study to evaluate the efficacy and safety of pembrolizumab plus BCG in HR NMIBC that is persistent/recurrent after BCG induction is currently open to enrollment (KEYNOTE-676, NCT03711032).
Presented by: Arjun V. Balar, MD, Director of the genitourinary medical oncology program at NYU Langone’s Perlmutter Cancer Center. New York, New York
Co-Authors: Girish S. Kulkarni, Edward M. Uchio, Joost Boormans, Loic Mourey, Laurence Eliot Miles Krieger, Eric A. Singer, Dean F. Bajorin, Ashish M. Kamat, Petros Grivas, Ho Kyung Seo, Hiroyuki Nishiyama, Badrinath R. Konety, Kijoeng Nam, Ekta Kapadia, Tara L. Frenkl, Ronald De Wit; UHN Princess Margaret Cancer Center, University of Toronto, Toronto, ON, Canada; UC Irvine Health, Orange, CA; Erasmus University Medical Center, Rotterdam, Netherlands; Institut Universitaire du Cancer Toulouse Oncopole, Toulouse, France; Royal North Shore Hospital, Northern Cancer Institute, St. Leonards, NSW, Australia; Section of Urologic Oncology, Rutgers Cancer Institute of New Jersey, New Brunswick, NJ; Memorial Sloan Kettering Cancer Center, New York, NY; The University of Texas MD Anderson Cancer Center, Houston, TX; University of Washington, Seattle, WA; National Cancer Center, Goyang, Korea, Republic of (South); University of Tsukuba, Tsukuba, Japan; University of Minnesota, Minneapolis, MN; Merck & Co., Inc., Kenilworth, NJ
Written By: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the 2019 American Society of Clinical Oncology Genitourinary Cancers Symposium, (ASCO GU) #GU19, February 14-16, 2019 - San Francisco, CA
References:
- Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol 2017;18(11):1483-1492.
- Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med 2017;376(11):1015-1026.


