Ibrutinib is an irreversible inhibitor of BTK (Bruton’s tyrosine kinase), a critical component of the B-cell receptor and cytokine receptor pathway. Ibrutinib is currently used in the liquid malignancy space, for the treatment of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), chronic graft-versus-host disease, mantle cell lymphoma, marginal zone lymphoma, and Waldenström macroglobulinemia. It has been hypothesized that ibrutinib may be efficacious in solid tumors as well through an upregulation of anti-tumor immune response by inhibiting ITK, thereby shifting the balance between Th1 and Th2 cells.
This abstract describes the first 29 patients with advanced urothelial carcinoma who were treated on the open label phase 1B/2 study of ibrutinib plus weekly paclitaxel 80 mg/m2 in 21 day cycles. 25 patients received ibrutinib 840 mg/day and 4 patients received ibrutinib 560 mg/day. The primary endpoint of the study was progression free survival.
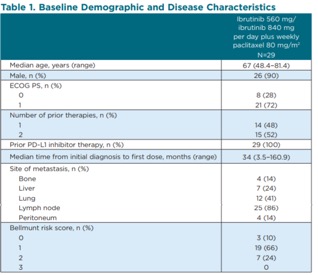
In terms of baseline characteristics, the median age was 67 and half of the patients had 2 prior lines of therapy. All patients had prior PD-L1 inhibitor treatment. 24% of patients had liver metastases, 41% of patients had lung metastases, and 14% of patients had bone metastases.
In terms of patient disposition, the median time on study treatment was 2.3 months. At the time of this interim analysis, 86% of patients had discontinued ibrutinib and 90% of patients had discontinued paclitaxel. The most common reason for discontinuation were progressive disease (62%), followed by adverse events (14%). 28% of patients required an ibrutinib dose reduction and paclitaxel dose reduction occurred in 52% of patients.
In terms of safety, the most frequent adverse events were diarrhea (79%), decreased appetite (55%), asthenia (45%), and fatigue (41%). Ibrutinib has anti-platelet activity and hematuria (24%) and epistaxis (21%) were observed and one patient had major hemorrhage.
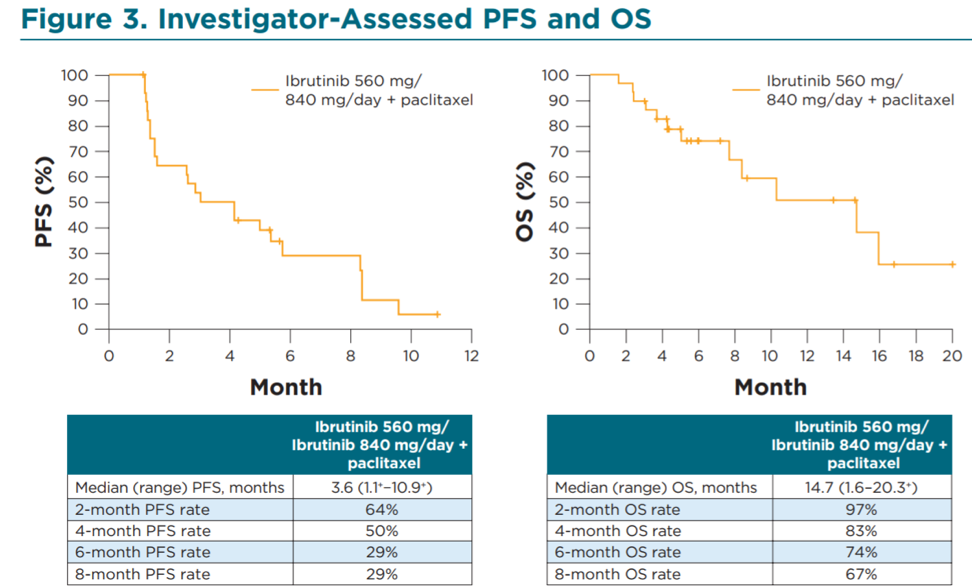

In the post immunotherapy setting, there are limited options for patients with metastatic urothelial carcinoma. In this study where 100% of patients had progressed on a checkpoint inhibitor, the combination of ibrutinib plus paclitaxel provided an impressive objective response rate of 41%. Unfortunately, the response was not durable as the majority of patients had progressed 3.6 months. Given the immunomodulatory effects of ibrutinib, perhaps adding on a checkpoint inhibitor may provide for a more durable response. Phase II studies in CLL are evaluating this strategy with ibrutinib plus nivolumab2.
Presented by: Daniel E. Castellano, MD; Madrid, Spain
Written by: Jason Zhu,MD, Fellow, Division of Hematology and Oncology, Duke University. Twitter: @TheRealJasonZhu at the 2019 American Society of Clinical Oncology Genitourinary Cancers Symposium, (ASCO GU) #GU19, February 14-16, 2019 - San Francisco, CA
References:
1. Bellmunt J, De Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. New England Journal of Medicine 2017;376:1015-26.
2. Jain N, Basu S, Thompson PA, et al. Nivolumab Combined with Ibrutinib for CLL and Richter Transformation: A Phase II Trial. Blood 2016;128:59.


