Pembrolizumab, a PD-1 inhibitor, has demonstrated significant activity and is FDA-approved for patients with advanced/chemotherapy-refractory metastatic urothelial carcinoma. The primary objective of this trial is to determine the overall survival (OS) and disease-free survival (DFS) in patients with high-risk MIBC treated with pembrolizumab after cystectomy. Secondary objectives will include assessment of DFS and OS in PD-L1-positive and -negative patients and assess the safety of this treatment.
Eligible patients must have a histologically confirmed MIBC or upper tract disease. Patients must have received NAC and have a pathologic stage of pT2 or above and N+, or be platinum ineligible, or decline adjuvant cisplatin-based or other systemic chemotherapy, and have a pathological stage at resection of pT3 or above, or pN+.
The study trial scheme is shown in figure 1. Patients will be stratified by pathologic stage, central PD-L1 status, and prior NAC. At trial accrual, patients will be randomized to receive pembrolizumab 240 mg every three weeks for one year, or observation. Lastly, quality of life will be assessed as well, and additional correlative objectives are shown in Figure 2. A total of 739 patients are planned to be accrued.
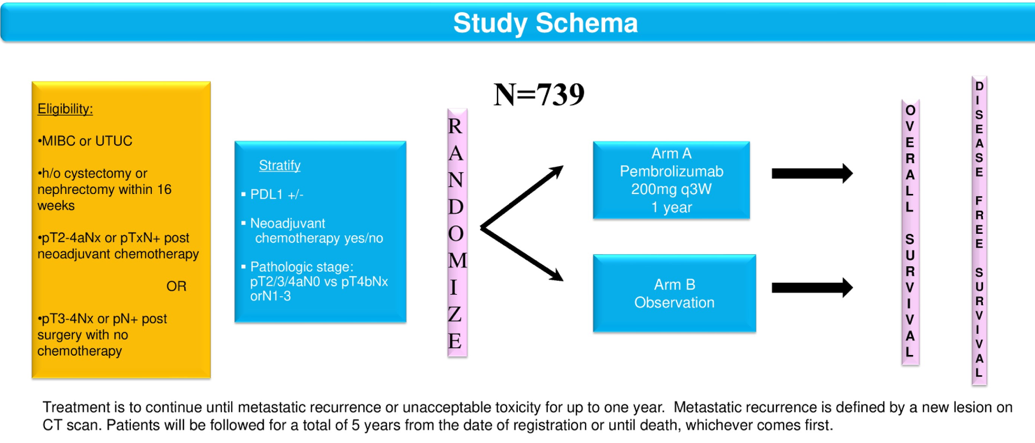
Figure 2 – Additional Correlative Objectives:

Presented by: Andrea Apolo, MD, Genitourinary Malignancies Branch, Center for Cancer Research, National Cancer Institute
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre @GoldbergHanan at the 2019 American Society of Clinical Oncology Genitourinary Cancers Symposium, (ASCO GU) #GU19, February 14-16, 2019 - San Francisco, CA


