TIVO-1 was a 1st line phase 3 RCC study of TIVO vs. Sorafenib. This showed superior progression-free survival (PFS) of 11.9 months vs. 9.1 months, HR 0.8, p=0.04. The objective response rate was 33% vs. 23%, and overall survival (OS) was 28.8 months vs. 29.3 months. This treatment received 1st line European Medical Association approval in August 2017.
The primary endpoint included PFS per independent review committee, with 90% power to detect PFS improvement of 4 vs. six months. The secondary endpoints included objective response rates, overall survival, investigator-assessed PFS, duration of response, and safety. Baseline characteristics are shown in table 1 and demonstrated matched and similar arms.

Figure 1 -TIVO-3 trial schema:
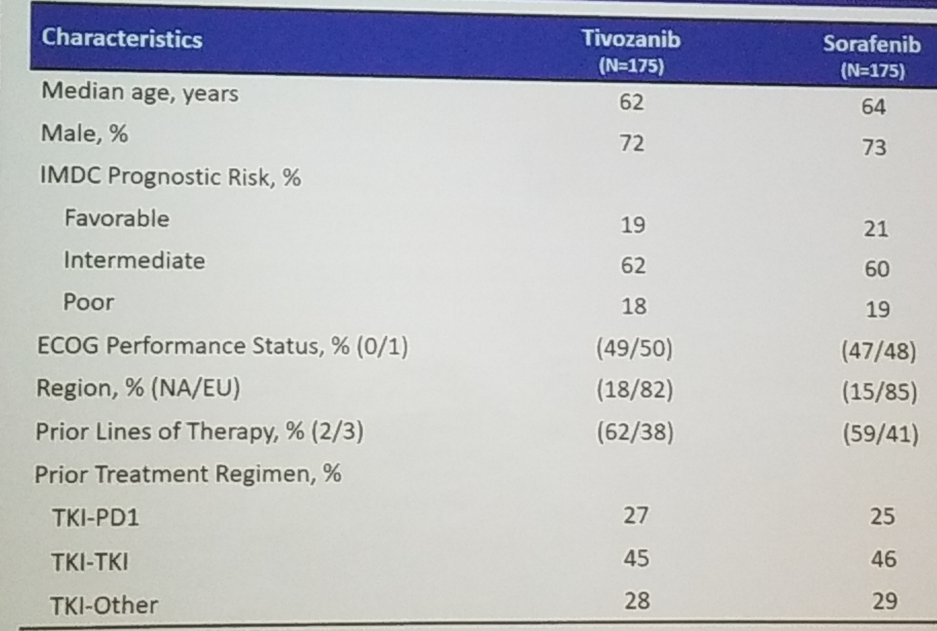
Table 1 – Study demographic detail:
Overall 175 patients have been assigned to each arm. The results demonstrated a clear advantage to the TIVO arm (Figure 2). The PFS results were similar when stratifying the results by previous immune checkpoint inhibitor therapy. Figure 3 demonstrates the overall survival data, showing no significant difference between the treatment arms. Currently, 19% and 5% of the patients in the TIVO and sorafenib arms, respectively, remain in their study arm. A total of 41% of the TIVO arm and 47% of the sorafenib arm have received subsequent therapies.
Figure 4 is a summary slide that demonstrates the treatment-related adverse events (>10% frequency in either arm).

Figure 2 – Progression free survival:

Figure 3 – Overall survival data:

Figure 4 – Treatment-related adverse events:
In conclusion, TIVO significantly improves PFS and objective response rates compared to sorafenib in patients with treatment-refractory advanced RCC. TIVO was also superior in patients previously treated with immune checkpoint inhibitors. TIVO was well-tolerated wit hypertension as the most common adverse event. Currently, the overall survival data are not mature enough, and further follow-up is required.
Presented by: Brian I. Rini, MD, FACP, Cleveland Clinic Taussig Cancer Institute, Cleveland, OH
Written By: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre (@GoldbergHanan) at the 2019 American Society of Clinical Oncology Genitourinary Cancers Symposium, (ASCO GU) #GU19, February 14-16, 2019 - San Francisco, CA


