This single-arm phase II trial will test the efficacy of nivolumab plus ipilimumab in up to 30 patients with SMARCB1-negative renal medullary carcinoma, renal cell carcinoma unclassified with medullary phenotype, and adult-onset kidney malignant rhabdoid tumors. The trial schema is as follows:
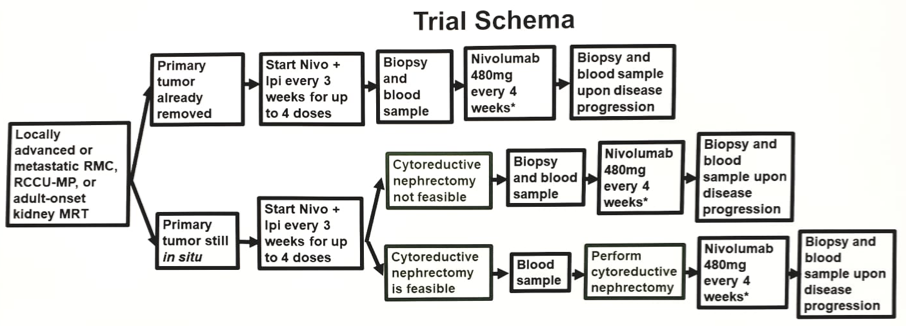
*Continued maintenance Nivo every 2 weeks until disease progression on unacceptable treatment-related toxicity
Any number of prior therapies is allowed and patients are eligible regardless if they’ve had a prior nephrectomy. Patients must not have had prior treatment with either anti-PD(L)1 or anti-CTLA-4 checkpoint inhibitors. Patients will be treated with ipilimumab1 mg/kg + nivolumab 3 mg/kg every 3 weeks x4 doses followed by maintenance nivolumab480 mg every 4 weeks for up to two years. Ongoing monitoring for safety and futility will be implemented using cohorts of 10 patients each. The primary endpoint is objective response rate and the trial objective is to achieve a similar or greater objective response rate compared with the historical objective response rate of 29% achieved at the MD Anderson Cancer Center using conventional cytotoxic chemotherapies. Secondary endpoints include progression-free survival, overall survival, and disease control rate. To evaluate potential biomarkers for treatment response, correlative analyses will be performed in tumor tissue and peripheral blood samples obtained at:
- Pre-treatment (baseline) up to 6 weeks (42 days) prior to initiation of treatment on Day 1
- After completion of the combination therapy (nivolumab plus ipilimumab) phase
- Upon disease progression.
At the time of the abstract submission, two patients have been enrolled in this study. Clinical trial information: NCT03274258
Presented by: Pavlos Msaouel, MD, Ph.D., The University of Texas MD Anderson Cancer Center, Houston, Texas
Written By: Gina B. Carithers Founder and Publisher UroToday.com
Zachary Klaassen, MD, MSc, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter:@zklaassen_md @ the 2019 American Society of Clinical Oncology Genitourinary Cancers Symposium, (ASCO GU) #GU19, February 14-16, 2019 - San Francisco, CA
References:
1. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med2018;378(14):1277-1290.
2. Beckermann KE, Jolly PC, Kim JY, et al. Clinical and immunologic correlates of response to PD-1 blockade in a patient with metastatic renal medullary carcinoma. J Immunother Cancer2017 Jan 17;5:1.
3. Sodji Q, Klein K, Sravan K, et al. Predictive role of PD-L1 expression in the response of renal medullary carcinoma to PD-1 inhibition.


